Eliglustat: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
m (Protected "Eliglustat": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (6 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=glucosylceramide synthase inhibitor | |drugClass=glucosylceramide synthase inhibitor | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=adult patients with Gaucher disease type 1 who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test | |indication=adult patients with [[Gaucher disease|Gaucher disease type 1]] who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test | ||
|adverseReactions=[[fatigue]], [[headache]], [[nausea]], [[diarrhea]], [[back pain]], pain in extremities, and | |adverseReactions=[[fatigue]], [[headache]], [[nausea]], [[diarrhea]], [[back pain]], pain in extremities, and [[abdominal pain|upper abdominal pain]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 16: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult===Indications== | |fdaLIADAdult===Indications== | ||
* | * Eliglustat is indicated for the long-term treatment of adult patients with [[Gaucher disease|Gaucher disease type 1]] (GD1) who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test. | ||
'''Limitations of Use''': | '''Limitations of Use''': | ||
* Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of | * Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of eliglustat to achieve a therapeutic effect . | ||
* A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers). | * A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers). | ||
| Line 32: | Line 32: | ||
'''Recommended Adult Dosage''' | '''Recommended Adult Dosage''' | ||
* The recommended dosage of | * The recommended dosage of eliglustat is 84 mg twice daily in CYP2D6 EMs and IMs. The recommended dosage in CYP2D6 PMs is 84 mg once daily; appropriate adverse event monitoring is recommended. The predicted exposures with 84 mg once daily in patients who are CYP2D6 PMs are expected to be similar to exposures observed with 84 mg twice daily in CYP2D6 IMs. | ||
* Some inhibitors of CYP2D6 and CYP3A are contraindicated with | * Some inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's metabolizer status. Co-administration of eliglustat with other CYP2D6 and CYP3A inhibitors may require dosage adjustment depending on the patient's CYP2D6 metabolizer status to reduce the risk of potentially significant adverse reactions. | ||
* Reduce the dosage of | * Reduce the dosage of eliglustat to 84 mg once daily for: | ||
:* CYP2D6 EMs and IMs taking strong or moderate CYP2D6 inhibitors | :* CYP2D6 EMs and IMs taking strong or moderate CYP2D6 inhibitors | ||
| Line 43: | Line 43: | ||
==DOSAGE FORMS AND STRENGTHS== | ==DOSAGE FORMS AND STRENGTHS== | ||
* | * Eliglustat is supplied as 84 mg hard gelatin capsules, with a pearl blue-green opaque cap and pearl white opaque body imprinted with "GZ02" in black. Each capsule contains 100 mg eliglustat tartrate, which is equivalent to 84 mg of eliglustat. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 53: | Line 53: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|contraindications=* | |contraindications=* Eliglustat is contraindicated in the following patients due to the risk of significantly increased eliglustat plasma concentrations which may result in prolongation of the PR, QTc, and/or QRS cardiac intervals that could result in cardiac arrhythmias. See TABLE 3 and TABLE 4 for examples of drugs in each of the categories described: | ||
:*EMs or IMs taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor. | :*EMs or IMs taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor. | ||
| Line 60: | Line 60: | ||
|warnings='''Drug-Drug Interactions''' | |warnings='''Drug-Drug Interactions''' | ||
* Eliglustat is a CYP2D6 and CYP3A substrate. Drugs that inhibit CYP2D6 and CYP3A metabolism pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac intervals that could result in cardiac arrhythmias. Some drugs that are inhibitors of CYP2D6 and CYP3A are contraindicated with | * Eliglustat is a CYP2D6 and CYP3A substrate. Drugs that inhibit CYP2D6 and CYP3A metabolism pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac intervals that could result in [[cardiac arrhythmias]]. Some drugs that are inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's CYP2D6 metabolizer status. | ||
'''ECG Changes and Potential for Cardiac Arrhythmias''' | '''ECG Changes and Potential for Cardiac Arrhythmias''' | ||
* Use of | * Use of eliglustat in patients with pre-existing cardiac conditions has not been studied during clinical trials. Because eliglustat is predicted to cause increases in ECG intervals (PR, QTc, and QRS) at substantially elevated eliglustat plasma concentrations, use of eliglustat is not recommended in patients with pre-existing cardiac disease ([[congestive heart failure]], recent [[myocardial infarction|acute myocardial infarction]], [[bradycardia]], [[heart block]], [[ventricular arrhythmia]]), [[long QT syndrome]], and in combination with Class IA (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications | ||
|clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
* The most common adverse reactions to | * The most common adverse reactions to eliglustat (occurring in ≥10% of the 126 GD1 patients treated with eliglustat across Trials 1 and 2) were [[fatigue]], [[headache]], [[nausea]], [[diarrhea]], [[back pain]], pain in extremities, and [[abdominal pain|upper abdominal pain]]. | ||
The adverse reaction profile of | The adverse reaction profile of eliglustat is based on two controlled studies, Trials 1 and 2. Table 1 presents the profile from the 9-month double-blind, randomized, placebo-controlled trial of 40 treatment-naïve patients (Trial 1). Patients were between the ages of 16 and 63 on the date of the first dose of study drug, and included 20 males and 20 females. | ||
[[File:Eliglustat table1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[File:Eliglustat table1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Table 2 presents the profile from the 12-month open-label, randomized, imiglucerase-controlled trial of 159 treated patients switching from enzyme replacement therapy (ERT) (Trial 2). Patients were between the ages of 18 and 69 on the date of the first dose of | * Table 2 presents the profile from the 12-month open-label, randomized, imiglucerase-controlled trial of 159 treated patients switching from enzyme replacement therapy (ERT) (Trial 2). Patients were between the ages of 18 and 69 on the date of the first dose of eliglustat, and included 87 females and 72 males. | ||
[[ File:Eliglustat table2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[ File:Eliglustat table2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 79: | Line 79: | ||
In an uncontrolled study, with up to 4 years of treatment, in 26 patients, the types and incidences of adverse reactions were similar to Trials 1 and 2. | In an uncontrolled study, with up to 4 years of treatment, in 26 patients, the types and incidences of adverse reactions were similar to Trials 1 and 2. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|drugInteractions====Potential for Other Drugs to Affect | |drugInteractions====Potential for Other Drugs to Affect Eliglustat=== | ||
Eliglustat is a CYP2D6 and CYP3A substrate. | Eliglustat is a CYP2D6 and CYP3A substrate. | ||
| Line 85: | Line 85: | ||
'''CYP2D6 and CYP3A Inhibitors''' | '''CYP2D6 and CYP3A Inhibitors''' | ||
* Drugs that inhibit CYP2D6 and CYP3A pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac interval which could result in cardiac arrhythmias: | * Drugs that inhibit CYP2D6 and CYP3A pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac interval which could result in [[cardiac arrhythmias]]: | ||
:*Some inhibitors of CYP2D6 and CYP3A are contraindicated with | :*Some inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's CYP2D6 metabolizer status. | ||
:*Co-administration of | :*Co-administration of eliglustat with other CYP2D6 and CYP3A inhibitors may require dosage adjustment depending on the patient's CYP2D6 metabolizer status to reduce the risk of potential significant adverse reactions. | ||
[[ File:Eliglustat table3.png |thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[ File:Eliglustat table3.png |thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 104: | Line 104: | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=* There is no dosage adjustment required for patients with mild renal impairment. | |useInRenalImpair=* There is no dosage adjustment required for patients with mild renal impairment. Eliglustat has not been studied in patients with moderate to severe renal impairment or end-stage renal disease (ESRD). Use of eliglustat in these patients is not recommended. | ||
|useInHepaticImpair=* | |useInHepaticImpair=* Eliglustat has not been studied in patients with hepatic impairment. Use of eliglustat is not recommended in all stages of hepatic impairment or cirrhosis. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
|overdose=* The highest eliglustat plasma concentration experienced to date occurred in a single-dose, dose escalation study in healthy subjects, in a subject taking a dose equivalent to approximately 21 times the recommended dose for GD1 patients. At the time of the highest plasma concentration (59-fold higher than normal therapeutic conditions), the subject experienced dizziness marked by disequilibrium, hypotension, bradycardia, nausea, and vomiting. | |overdose=* The highest eliglustat plasma concentration experienced to date occurred in a single-dose, dose escalation study in healthy subjects, in a subject taking a dose equivalent to approximately 21 times the recommended dose for GD1 patients. At the time of the highest plasma concentration (59-fold higher than normal therapeutic conditions), the subject experienced dizziness marked by disequilibrium, [[hypotension]], [[bradycardia]], [[nausea]], and [[vomiting]]. | ||
* In the event of acute overdose, the patient should be carefully observed and given symptomatic and supportive treatment. | * In the event of acute overdose, the patient should be carefully observed and given symptomatic and supportive treatment. | ||
| Line 120: | Line 117: | ||
* Hemodialysis is unlikely to be beneficial given that eliglustat has a large volume of distribution | * Hemodialysis is unlikely to be beneficial given that eliglustat has a large volume of distribution | ||
|drugBox=[[File:Eliglustat drug box.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |drugBox=[[File:Eliglustat drug box.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
|mechAction=* Gaucher disease is caused by a deficiency of the lysosomal enzyme acid β-glucosidase. Acid β-glucosidase catalyzes the conversion of the sphingolipid glucocerebroside into glucose and ceramide. The enzymatic deficiency causes an accumulation of glucosylceramide (GL-1) primarily in the lysosomal compartment of macrophages, giving rise to foam cells or "Gaucher cells". | |mechAction=* Gaucher disease is caused by a deficiency of the lysosomal enzyme acid β-glucosidase. Acid β-glucosidase catalyzes the conversion of the sphingolipid glucocerebroside into glucose and ceramide. The enzymatic deficiency causes an accumulation of glucosylceramide (GL-1) primarily in the lysosomal compartment of macrophages, giving rise to foam cells or "Gaucher cells". Eliglustat is a specific inhibitor of glucosylceramide synthase (IC50 = 10 ng/mL), and acts as a substrate reduction therapy for GD1. In clinical trials eliglustat reduced spleen and liver size, and improved [[anemia]] and [[thrombocytopenia]]. | ||
* In this lysosomal storage disorder (LSD), clinical features are reflective of the accumulation of Gaucher cells in the liver, spleen, bone marrow, and other organs. The accumulation of Gaucher cells in the liver, spleen, and bone marrow leads to organomegaly and skeletal disease. Presence of Gaucher cells in the bone marrow and spleen lead to clinically significant anemia and [[thrombocytopenia]]. | * In this lysosomal storage disorder (LSD), clinical features are reflective of the accumulation of Gaucher cells in the liver, spleen, bone marrow, and other organs. The accumulation of Gaucher cells in the liver, spleen, and bone marrow leads to organomegaly and skeletal disease. Presence of Gaucher cells in the bone marrow and spleen lead to clinically significant anemia and [[thrombocytopenia]]. | ||
| Line 135: | Line 132: | ||
'''Absorption''' | '''Absorption''' | ||
* In CYP2D6 EMs, median time to reach maximum plasma concentrations (tmax) occurs at 1.5 to 2 hours following multiple doses of | * In CYP2D6 EMs, median time to reach maximum plasma concentrations (tmax) occurs at 1.5 to 2 hours following multiple doses of eliglustat 84 mg twice daily. The corresponding mean Cmax values range from 12.1 to 25.0 ng/mL in EMs. The mean AUCtau values range from 76.3 to 143 hr*ng/mL in EMs. The Cmax and AUCtau in one IM subject receiving multiple doses of eliglustat 84 mg two time daily was 44.6 ng/mL and 306 hr*ng/mL, respectively. The oral bioavailability is low in EMs (<5%) following single dose of eliglustat 84 mg due to significant first-pass metabolism. | ||
* In PMs, median tmax occurs at 3 hours following multiple doses of | * In PMs, median tmax occurs at 3 hours following multiple doses of eliglustat 84 mg twice daily. The corresponding mean Cmax and AUCtau values range from 113 to 137 ng/mL and 922 to 1057 hr*ng/mL, respectively. | ||
Oral dosing of | * Oral dosing of eliglustat 84 mg once daily has not been studied in PMs. The predicted Cmax and AUC0-24hr in PMs using physiologically-based pharmacokinetic (PBPK) model with 84 mg once daily are 75 ng/mL and 956 hr*ng/mL, respectively. | ||
* Administration of | * Administration of eliglustat with a high fat meal resulted in a 15% decrease in Cmax but no change in AUC. Food does not have a clinically relevant effect on eliglustat pharmacokinetics. | ||
'''Distribution''' | '''Distribution''' | ||
* Eliglustat is moderately bound to human plasma proteins (76 to 83%). In the blood, it is mainly distributed in plasma and not red blood cells. After intravenous (IV) administration, the volume of distribution of eliglustat was 835 L in CYP2D6 EMs, suggesting wide distribution to tissues ( | * Eliglustat is moderately bound to human plasma proteins (76 to 83%). In the blood, it is mainly distributed in plasma and not red blood cells. After intravenous (IV) administration, the volume of distribution of eliglustat was 835 L in CYP2D6 EMs, suggesting wide distribution to tissues (eliglustat is only for oral use). | ||
'''Metabolism and Elimination''' | '''Metabolism and Elimination''' | ||
* | * Eliglustat is extensively metabolized with high clearance, mainly by CYP2D6 and to a lesser extent CYP3A4. Primary metabolic pathways of eliglustat involve sequential oxidation of the octanoyl moiety followed by oxidation of the 2,3-dihydro-1,4-benzodioxane moiety, or a combination of the two pathways, resulting in multiple oxidative metabolites. No active metabolites have been identified. | ||
* After oral administration of 84 mg [14C]-eliglustat, the majority of the administered dose is excreted in urine (41.8%) and feces (51.4%), mainly as metabolites. After 42 mg IV administration in healthy volunteers, mean (CV%) of eliglustat total body clearance was 88 L/h (8.8%) in CYP2D6 EMs ( | * After oral administration of 84 mg [14C]-eliglustat, the majority of the administered dose is excreted in urine (41.8%) and feces (51.4%), mainly as metabolites. After 42 mg IV administration in healthy volunteers, mean (CV%) of eliglustat total body clearance was 88 L/h (8.8%) in CYP2D6 EMs (eliglustat is only for oral use). Following multiple oral doses of eliglustat 84 mg twice daily, eliglustat terminal elimination half-life (T1/2) was approximately 6.5 hours in EMs and 8.9 hours in PMs. | ||
'''Specific Populations''' | '''Specific Populations''' | ||
| Line 157: | Line 154: | ||
* Based on population PK analysis, there was no effect of mild renal impairment on eliglustat PK. Furthermore, gender, body weight, age, and race had no clinically relevant impact on the pharmacokinetics of eliglustat. | * Based on population PK analysis, there was no effect of mild renal impairment on eliglustat PK. Furthermore, gender, body weight, age, and race had no clinically relevant impact on the pharmacokinetics of eliglustat. | ||
'''Drug Interactions''' - Effect of Other Drugs on | '''Drug Interactions''' - Effect of Other Drugs on Eliglustat | ||
* In vitro, eliglustat is metabolized primarily by CYP2D6 and to a lesser extent by CYP3A4. Eliglustat is also a substrate of P-glycoprotein (P-gp). | * In vitro, eliglustat is metabolized primarily by CYP2D6 and to a lesser extent by CYP3A4. Eliglustat is also a substrate of P-glycoprotein (P-gp). | ||
* Co-administration of | * Co-administration of eliglustat with CYP2D6 Inhibitors | ||
* Systemic exposure (Cmax and AUCtau) of eliglustat increased 7.0-fold and 8.4-fold, respectively, following co-administration of | * Systemic exposure (Cmax and AUCtau) of eliglustat increased 7.0-fold and 8.4-fold, respectively, following co-administration of eliglustat 84 mg twice daily with paroxetine (a strong CYP2D6 inhibitor) 30 mg once daily in EMs (N=30), respectively. | ||
* Simulations using PBPK models suggested that paroxetine may increase the Cmax and AUCtau of eliglustat 2.1- and 2.3-fold in IMs, respectively. | * Simulations using PBPK models suggested that paroxetine may increase the Cmax and AUCtau of eliglustat 2.1- and 2.3-fold in IMs, respectively. | ||
| Line 169: | Line 166: | ||
* Compared to paroxetine, the effects of terbinafine (a moderate inhibitor of CYP2D6) on the exposure of eliglustat in EMs or IMs were predicted to be smaller. Simulations using PBPK models suggested that terbinafine may increase the Cmax and AUCtau of eliglustat 3.8- and 4.5-fold in EMs, respectively. Both Cmax and AUCtau increased 1.6-fold in IMs. | * Compared to paroxetine, the effects of terbinafine (a moderate inhibitor of CYP2D6) on the exposure of eliglustat in EMs or IMs were predicted to be smaller. Simulations using PBPK models suggested that terbinafine may increase the Cmax and AUCtau of eliglustat 3.8- and 4.5-fold in EMs, respectively. Both Cmax and AUCtau increased 1.6-fold in IMs. | ||
''Co-administration of | ''Co-administration of Eliglustat with CYP3A Inhibitors'' | ||
''CYP2D6 EMs and IMs'': | ''CYP2D6 EMs and IMs'': | ||
* Following co-administration of | * Following co-administration of eliglustat 84 mg twice daily with ketoconazole (a strong CYP3A inhibitor) 400 mg once daily, the systemic exposure (Cmax and AUCtau) of eliglustat increased 4.0-fold and 4.4-fold in EMs (N=31). | ||
* Simulations using PBPK models suggested that ketoconazole may increase the Cmax and AUCtau of eliglustat 4.4- and 5.4-fold in IMs, respectively. | * Simulations using PBPK models suggested that ketoconazole may increase the Cmax and AUCtau of eliglustat 4.4- and 5.4-fold in IMs, respectively. | ||
| Line 181: | Line 178: | ||
''CYP2D6 PMs'': | ''CYP2D6 PMs'': | ||
* The effect of CYP3A inhibitors on the systemic exposure of eliglustat in PMs has not been evaluated in clinical studies. Simulations using PBPK models suggest that ketoconazole may increase the Cmax and AUC0-24h of eliglustat 4.3- and 6.2-fold when co-administered with | * The effect of CYP3A inhibitors on the systemic exposure of eliglustat in PMs has not been evaluated in clinical studies. Simulations using PBPK models suggest that ketoconazole may increase the Cmax and AUC0-24h of eliglustat 4.3- and 6.2-fold when co-administered with eliglustat 84 mg once daily in PMs. Simulations using PBPK models suggested that fluconazole may increase the Cmax and AUC0-24h of eliglustat 2.4- and 3.0-fold, respectively, when co-administered with eliglustat 84 mg once daily. | ||
''Co-administration of | ''Co-administration of Eliglustat with CYP2D6 and CYP3A inhibitors'' | ||
* Simulations using PBPK models suggested that concomitant use of | * Simulations using PBPK models suggested that concomitant use of eliglustat 84 mg twice daily with paroxetine and ketoconazole may increase the Cmax and AUCtau of eliglustat 16.7- and 24.2-fold in EMs, respectively. The predicted Cmax and AUCtau of eliglustat increased 7.5- to 9.8-fold in IMs, respectively. | ||
* Simulations using PBPK models suggested that concomitant use of | * Simulations using PBPK models suggested that concomitant use of eliglustat 84 mg twice daily with terbinafine and fluconazole may increase the Cmax and AUCtau of eliglustat 10.2- and 13.6-fold in EMs. The predicted Cmax and AUCtau of eliglustat increased 4.2- to 5.0-fold in IMs, respectively. | ||
''Effect of CYP3A inducers on Eliglustat PK'' | ''Effect of CYP3A inducers on Eliglustat PK'' | ||
* Systemic exposures (Cmax and AUCtau) of eliglustat decreased by approximately 90% in EMs and IMs, following co-administration of | * Systemic exposures (Cmax and AUCtau) of eliglustat decreased by approximately 90% in EMs and IMs, following co-administration of eliglustat 127 mg twice daily with rifampin (a strong CYP3A inducer) 600 mg PO once daily. The only approved dose of eliglustat is 84 mg. Systemic exposures of eliglustat decreased by approximately 95% following co-administration of eliglustat 84 mg twice daily with rifampin 600 mg PO once daily in PMs. | ||
''Effect of OATP (organic anion transporting polypeptide) Inhibitors on Eliglustat PK'' | ''Effect of OATP (organic anion transporting polypeptide) Inhibitors on Eliglustat PK'' | ||
| Line 205: | Line 202: | ||
* Gastric pH-modifying agents (Maalox®, Tums®, Protonix®) did not have a clinically relevant effect on eliglustat exposure. | * Gastric pH-modifying agents (Maalox®, Tums®, Protonix®) did not have a clinically relevant effect on eliglustat exposure. | ||
''Drug Interactions - Effect of | ''Drug Interactions - Effect of eliglustat on the PK of Other Drugs'' | ||
* Eliglustat is an inhibitor of P-gp and CYP2D6. | * Eliglustat is an inhibitor of P-gp and CYP2D6. | ||
* Following multiple doses of | * Following multiple doses of eliglustat 127 mg twice daily, systemic exposures (Cmax and AUC) to metoprolol (a CYP2D6 substrate) increased compared to metoprolol administration alone. Mean Cmax and AUC increased by 1.7- and 2.3-fold, respectively, in EMs and by 1.2- and 1.6-fold, respectively in IMs. The only approved dose of eliglustat is 84 mg. | ||
* Following multiple doses of | * Following multiple doses of eliglustat 127 mg twice daily in EMs and IMs or 84 mg twice daily in PMs, systemic exposures (Cmax and AUC) to digoxin (a P-gp substrate, with narrow therapeutic index) increased compared to digoxin administration alone. Mean Cmax and AUC increased by 1.7- and 1.5-fold, respectively. The only approved dose of eliglustat is 84 mg. | ||
* In vitro, eliglustat is a weak inhibitor of CYP3A. Repeated doses of | * In vitro, eliglustat is a weak inhibitor of CYP3A. Repeated doses of eliglustat 84 mg twice daily did not change the exposures to norethindrone (1.0 mg) and ethinyl estradiol (0.035 mg). Therefore, eliglustat is not expected to impact the efficacy or safety of oral contraceptives containing norethindrone and ethinyl estradiol. | ||
|nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | |nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | ||
'''Carcinogenesis''' | '''Carcinogenesis''' | ||
* Carcinogenic potential of | * Carcinogenic potential of eliglustat was assessed in 2-year carcinogenicity studies in rats and mice. In Sprague-Dawley rats, eliglustat was administered by oral gavage at doses up to 75 mg/kg/day in males (about 3.6 times the recommended human daily dose of 84 mg twice daily, based on body surface area) and 50 mg/kg/day in females (about 2.4 times the recommended human daily dose based on body surface area). In CD-1 mice, eliglustat was administered to males and females at up to 75 mg/kg/day (about 1.8 times the recommended human daily dose based on body surface area) via dietary admixture. Eliglustat did not produce any treatment-related neoplasms in rats or mice. | ||
'''Mutagenesis''' | '''Mutagenesis''' | ||
| Line 229: | Line 226: | ||
* In mature male rats, eliglustat showed reversible adverse effects on sperm morphology, testes (germ cell necrosis), and sloughed cells in the epididymis at 200 mg/kg/day (about 10 times the recommended human oral dose based on body surface area). Similar effects on sperm were not seen in mature Cynomolgus monkeys at 72 mg/kg/day (about 7 times the recommended human oral dose based on body surface area). | * In mature male rats, eliglustat showed reversible adverse effects on sperm morphology, testes (germ cell necrosis), and sloughed cells in the epididymis at 200 mg/kg/day (about 10 times the recommended human oral dose based on body surface area). Similar effects on sperm were not seen in mature Cynomolgus monkeys at 72 mg/kg/day (about 7 times the recommended human oral dose based on body surface area). | ||
|clinicalStudies=* The efficacy of | |clinicalStudies=* The efficacy of eliglustat was evaluated in three clinical trials in patients with Gaucher disease type 1. | ||
=== | ===Eliglustat in Treatment-Naïve GD1 Patients – Trial 1=== | ||
* Trial 1 was a randomized, double-blind, placebo-controlled, multicenter clinical study evaluating the efficacy and safety of | * Trial 1 was a randomized, double-blind, placebo-controlled, multicenter clinical study evaluating the efficacy and safety of eliglustat in 40 treatment-naïve GD1 patients 16 years of age or older (median age 30.4 years) with pre-existing splenomegaly and hematological abnormalities. Patients were required to have received no treatment with substrate reduction therapy within 6 months or ERT within 9 months prior to randomization; all but 5 patients in the study had no prior therapy. Patients were stratified according to baseline spleen volume (≤ 20 or > 20 multiples of normal [MN]) and randomized in a 1:1 ratio to receive eliglustat or placebo for the duration of the 9-month blinded primary analysis period. The eliglustat treatment group was comprised of IM (5%), EM (90%) and URM (5%) patients. Patients randomized to eliglustat treatment received a starting dose of 42 mg twice daily, with a dose increase to 84 mg twice daily possible at Week 4 based on the plasma trough concentration at Week 2. The majority of patients (17 [85%]) received a dose escalation to 84 mg twice daily at Week 4, and 3 (15%) continued to receive 42 mg twice daily for the duration of the 9-month blinded primary analysis period. | ||
* The primary endpoint was the percentage change in spleen volume (in MN) from baseline to 9 months as compared to placebo. Secondary endpoints were absolute change in hemoglobin level, percentage change in liver volume (in MN), and percentage change in platelet count from baseline to 9 months compared to placebo. | * The primary endpoint was the percentage change in spleen volume (in MN) from baseline to 9 months as compared to placebo. Secondary endpoints were absolute change in hemoglobin level, percentage change in liver volume (in MN), and percentage change in platelet count from baseline to 9 months compared to placebo. | ||
* At baseline, mean spleen volumes were 12.5 and 13.9 MN in the placebo and | * At baseline, mean spleen volumes were 12.5 and 13.9 MN in the placebo and eliglustat groups, respectively, and mean liver volumes were 1.4 MN for both groups. Mean hemoglobin levels were 12.8 and 12.1 g/dL, and platelet counts were 78.5 and 75.1 x 109/L, respectively. | ||
* During the 9-month primary analysis period, | * During the 9-month primary analysis period, eliglustat demonstrated statistically significant improvements in all primary and secondary endpoints compared to placebo, as shown in Table 6. | ||
[[File:Eliglustat table6.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[File:Eliglustat table6.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 245: | Line 242: | ||
* In an uncontrolled study of treatment naïve GD1 patients, improvements in spleen and liver volume, hemoglobin level, and platelet count continued through the 4 year treatment period. | * In an uncontrolled study of treatment naïve GD1 patients, improvements in spleen and liver volume, hemoglobin level, and platelet count continued through the 4 year treatment period. | ||
===Patients Switching from Enzyme Replacement Therapy to | ===Patients Switching from Enzyme Replacement Therapy to Eliglustat – Trial 2=== | ||
* Trial 2 was a randomized, open-label, active-controlled, non-inferiority, multicenter clinical study evaluating the efficacy and safety of | * Trial 2 was a randomized, open-label, active-controlled, non-inferiority, multicenter clinical study evaluating the efficacy and safety of eliglustat compared with imiglucerase in 159 treated GD1 patients (median age 37.4 years) previously treated with enzyme replacement therapy (≥3 years of enzyme replacement therapy, dosed at 30-130 U/kg/month in at least 6 of the prior 9 months) who met pre-specified therapeutic goals at baseline. Pre-specified baseline therapeutic goals included: no bone crisis and free of symptomatic bone disease within the last year; mean hemoglobin level of ≥ 11 g/dL in females and ≥ 12 g/dL in males; mean platelet count ≥ 100,000/mm3; spleen volume < 10 times normal and liver volume < 1.5 times normal. | ||
* Patients were randomized 2:1 to receive | * Patients were randomized 2:1 to receive eliglustat or imiglucerase for the duration of the 12-month primary analysis period. Seventy-five percent of patients randomized to eliglustat were previously treated with imiglucerase; 21% with velaglucerase alfa and 4% were unreported. Patients randomized to eliglustat treatment received a starting dose of 42 mg twice daily, with dose increases to 84 mg twice daily and 127 mg twice daily possible at Weeks 4 and 8 based on plasma trough concentrations of eliglustat at Weeks 2 and 6, respectively. The percentage of patients receiving the 3 possible eliglustat doses was: 42 mg twice daily (20%), 84 mg twice daily (32%) and 127 mg twice daily (48%). The eliglustat treatment group was comprised of PM (4%), IM (10%), EM (80%) and URM (4%) patients. | ||
* At baseline, mean spleen volumes were 2.6 and 3.2 MN in the imiglucerase and | * At baseline, mean spleen volumes were 2.6 and 3.2 MN in the imiglucerase and eliglustat groups, respectively, and liver volumes were 0.9 MN in both groups. Mean hemoglobin levels were 13.8 and 13.6 g/dL, and platelet counts were 192 and 207 x 109/L, respectively. | ||
* The primary composite endpoint required stability in all four component domains (hemoglobin level, platelet count, liver volume, and spleen volume) based on changes between baseline and 12 months. Stability was defined by the following pre-specified thresholds of change: hemoglobin level <1.5 g/dL decrease, platelet count < 25% decrease, liver volume <20% increase and spleen volume <25% increase. The percentages of patients meeting the criteria for stability in the individual components of the composite endpoint were assessed as secondary efficacy endpoints. | * The primary composite endpoint required stability in all four component domains (hemoglobin level, platelet count, liver volume, and spleen volume) based on changes between baseline and 12 months. Stability was defined by the following pre-specified thresholds of change: hemoglobin level <1.5 g/dL decrease, platelet count < 25% decrease, liver volume <20% increase and spleen volume <25% increase. The percentages of patients meeting the criteria for stability in the individual components of the composite endpoint were assessed as secondary efficacy endpoints. | ||
* | * Eliglustat met the criteria to be declared non-inferior to imiglucerase in maintaining patient stability. After 12 months of treatment, the percentage of patients meeting the primary composite endpoint was 84.8% for the eliglustat group compared to 93.6% for the imiglucerase group. The lower bound of the 95% CI of the 8.8% difference, -17.6%, was within the pre-specified non-inferiority margin of -25%. At Month 12, the percentages of CERDELGA and imiglucerase patients respectively, who met stability criteria for the individual components of the composite endpoint were: hemoglobin level, 94.9% and 100%; platelet count, 92.9% and 100%; spleen volume, 95.8% and 100%; and liver volume, 96.0% and 93.6%. Of the patients who did not meet stability criteria for the individual components, 12 of 15 CERDELGA patients and 3 of 3 imiglucerase patients remained within therapeutic goals for GD1. | ||
* Mean changes from baseline in the hematological and visceral parameters through 12 months of treatment are shown in Table 7. There were no clinically meaningful differences between groups for any of the four parameters. | * Mean changes from baseline in the hematological and visceral parameters through 12 months of treatment are shown in Table 7. There were no clinically meaningful differences between groups for any of the four parameters. | ||
[[File:Eliglustat table7.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | [[File:Eliglustat table7.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
|howSupplied=* | |howSupplied=* Eliglustat is supplied as 84 mg hard gelatin capsules, with a pearl blue-green opaque cap and pearl white opaque body imprinted with "GZ02" in black. | ||
* | * Eliglustat 84 mg capsules are supplied as: | ||
NDC-58468-0220-1 – Carton containing 4 packs of capsules (56 capsules total). Each pack is composed of 1 blister card of 14 capsules and a cardboard wallet. | NDC-58468-0220-1 – Carton containing 4 packs of capsules (56 capsules total). Each pack is composed of 1 blister card of 14 capsules and a cardboard wallet. | ||
| Line 295: | Line 292: | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* CERDELGA | |brandNames=* CERDELGA | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
| Line 310: | Line 306: | ||
|fileName=Eliglustat medication guide.png | |fileName=Eliglustat medication guide.png | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
| Line 322: | Line 317: | ||
<!--Category--> | <!--Category--> | ||
[[Category:Carboxamides]] | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 20:21, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Eliglustat is a glucosylceramide synthase inhibitor that is FDA approved for the treatment of adult patients with Gaucher disease type 1 who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test. Common adverse reactions include fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Eliglustat is indicated for the long-term treatment of adult patients with Gaucher disease type 1 (GD1) who are CYP2D6 extensive metabolizers (EMs), intermediate metabolizers (IMs), or poor metabolizers (PMs) as detected by an FDA-cleared test.
Limitations of Use:
- Patients who are CYP2D6 ultra-rapid metabolizers (URMs) may not achieve adequate concentrations of eliglustat to achieve a therapeutic effect .
- A specific dosage cannot be recommended for those patients whose CYP2D6 genotype cannot be determined (indeterminate metabolizers).
Dosage
Patient Selection
- Select patients with Gaucher disease type 1 based on their CYP2D6 metabolizer status. It is recommended patient genotypes be established using an FDA-cleared test for determining CYP2D6 genotype.
Recommended Adult Dosage
- The recommended dosage of eliglustat is 84 mg twice daily in CYP2D6 EMs and IMs. The recommended dosage in CYP2D6 PMs is 84 mg once daily; appropriate adverse event monitoring is recommended. The predicted exposures with 84 mg once daily in patients who are CYP2D6 PMs are expected to be similar to exposures observed with 84 mg twice daily in CYP2D6 IMs.
- Some inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's metabolizer status. Co-administration of eliglustat with other CYP2D6 and CYP3A inhibitors may require dosage adjustment depending on the patient's CYP2D6 metabolizer status to reduce the risk of potentially significant adverse reactions.
- Reduce the dosage of eliglustat to 84 mg once daily for:
- CYP2D6 EMs and IMs taking strong or moderate CYP2D6 inhibitors
- CYP2D6 EMs taking strong or moderate CYP3A inhibitors
DOSAGE FORMS AND STRENGTHS
- Eliglustat is supplied as 84 mg hard gelatin capsules, with a pearl blue-green opaque cap and pearl white opaque body imprinted with "GZ02" in black. Each capsule contains 100 mg eliglustat tartrate, which is equivalent to 84 mg of eliglustat.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eliglustat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eliglustat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Eliglustat in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eliglustat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eliglustat in pediatric patients.
Contraindications
- Eliglustat is contraindicated in the following patients due to the risk of significantly increased eliglustat plasma concentrations which may result in prolongation of the PR, QTc, and/or QRS cardiac intervals that could result in cardiac arrhythmias. See TABLE 3 and TABLE 4 for examples of drugs in each of the categories described:
- EMs or IMs taking a strong or moderate CYP2D6 inhibitor concomitantly with a strong or moderate CYP3A inhibitor.
- IMs or PMs taking a strong CYP3A inhibitor.
Warnings
Drug-Drug Interactions
- Eliglustat is a CYP2D6 and CYP3A substrate. Drugs that inhibit CYP2D6 and CYP3A metabolism pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac intervals that could result in cardiac arrhythmias. Some drugs that are inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's CYP2D6 metabolizer status.
ECG Changes and Potential for Cardiac Arrhythmias
- Use of eliglustat in patients with pre-existing cardiac conditions has not been studied during clinical trials. Because eliglustat is predicted to cause increases in ECG intervals (PR, QTc, and QRS) at substantially elevated eliglustat plasma concentrations, use of eliglustat is not recommended in patients with pre-existing cardiac disease (congestive heart failure, recent acute myocardial infarction, bradycardia, heart block, ventricular arrhythmia), long QT syndrome, and in combination with Class IA (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The most common adverse reactions to eliglustat (occurring in ≥10% of the 126 GD1 patients treated with eliglustat across Trials 1 and 2) were fatigue, headache, nausea, diarrhea, back pain, pain in extremities, and upper abdominal pain.
The adverse reaction profile of eliglustat is based on two controlled studies, Trials 1 and 2. Table 1 presents the profile from the 9-month double-blind, randomized, placebo-controlled trial of 40 treatment-naïve patients (Trial 1). Patients were between the ages of 16 and 63 on the date of the first dose of study drug, and included 20 males and 20 females.
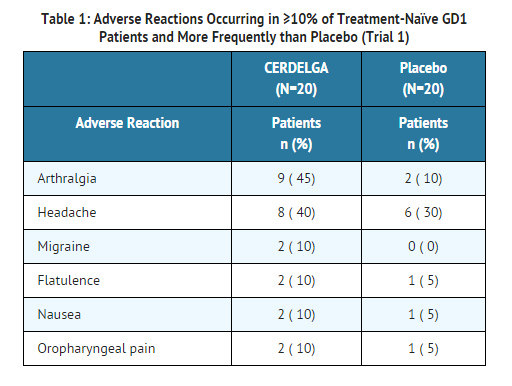
- Table 2 presents the profile from the 12-month open-label, randomized, imiglucerase-controlled trial of 159 treated patients switching from enzyme replacement therapy (ERT) (Trial 2). Patients were between the ages of 18 and 69 on the date of the first dose of eliglustat, and included 87 females and 72 males.
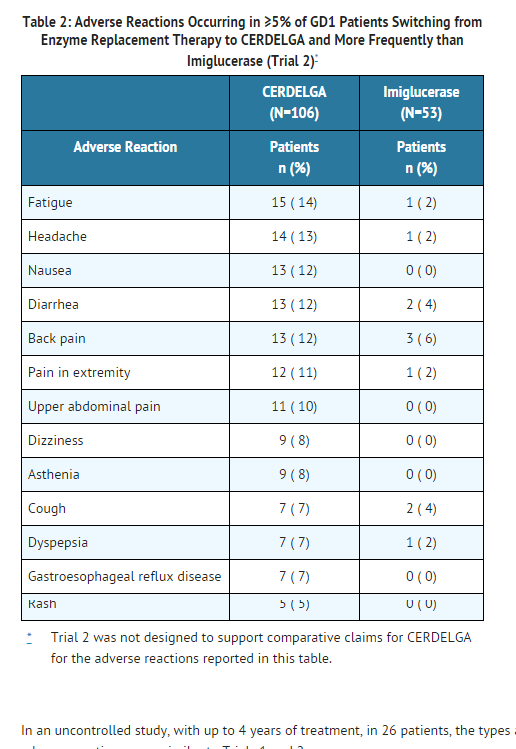
In an uncontrolled study, with up to 4 years of treatment, in 26 patients, the types and incidences of adverse reactions were similar to Trials 1 and 2.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Eliglustat in the drug label.
Drug Interactions
Potential for Other Drugs to Affect Eliglustat
Eliglustat is a CYP2D6 and CYP3A substrate.
CYP2D6 and CYP3A Inhibitors
- Drugs that inhibit CYP2D6 and CYP3A pathways may significantly increase the exposure to eliglustat and result in prolongation of the PR, QTc, and/or QRS cardiac interval which could result in cardiac arrhythmias:
- Some inhibitors of CYP2D6 and CYP3A are contraindicated with eliglustat depending on the patient's CYP2D6 metabolizer status.
- Co-administration of eliglustat with other CYP2D6 and CYP3A inhibitors may require dosage adjustment depending on the patient's CYP2D6 metabolizer status to reduce the risk of potential significant adverse reactions.
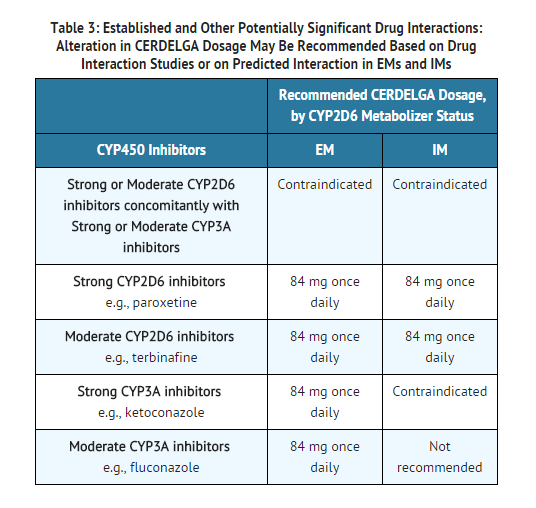
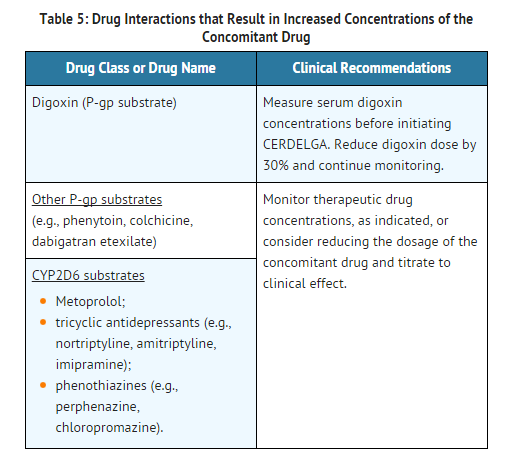
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Eliglustat in women who are pregnant.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eliglustat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eliglustat during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Eliglustat with respect to nursing mothers.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Eliglustat with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Eliglustat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Eliglustat with respect to specific racial populations.
Renal Impairment
- There is no dosage adjustment required for patients with mild renal impairment. Eliglustat has not been studied in patients with moderate to severe renal impairment or end-stage renal disease (ESRD). Use of eliglustat in these patients is not recommended.
Hepatic Impairment
- Eliglustat has not been studied in patients with hepatic impairment. Use of eliglustat is not recommended in all stages of hepatic impairment or cirrhosis.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Eliglustat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Eliglustat in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Eliglustat in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Eliglustat in the drug label.
Overdosage
- The highest eliglustat plasma concentration experienced to date occurred in a single-dose, dose escalation study in healthy subjects, in a subject taking a dose equivalent to approximately 21 times the recommended dose for GD1 patients. At the time of the highest plasma concentration (59-fold higher than normal therapeutic conditions), the subject experienced dizziness marked by disequilibrium, hypotension, bradycardia, nausea, and vomiting.
- In the event of acute overdose, the patient should be carefully observed and given symptomatic and supportive treatment.
- Hemodialysis is unlikely to be beneficial given that eliglustat has a large volume of distribution
Pharmacology
Mechanism of Action
- Gaucher disease is caused by a deficiency of the lysosomal enzyme acid β-glucosidase. Acid β-glucosidase catalyzes the conversion of the sphingolipid glucocerebroside into glucose and ceramide. The enzymatic deficiency causes an accumulation of glucosylceramide (GL-1) primarily in the lysosomal compartment of macrophages, giving rise to foam cells or "Gaucher cells". Eliglustat is a specific inhibitor of glucosylceramide synthase (IC50 = 10 ng/mL), and acts as a substrate reduction therapy for GD1. In clinical trials eliglustat reduced spleen and liver size, and improved anemia and thrombocytopenia.
- In this lysosomal storage disorder (LSD), clinical features are reflective of the accumulation of Gaucher cells in the liver, spleen, bone marrow, and other organs. The accumulation of Gaucher cells in the liver, spleen, and bone marrow leads to organomegaly and skeletal disease. Presence of Gaucher cells in the bone marrow and spleen lead to clinically significant anemia and thrombocytopenia.
Structure
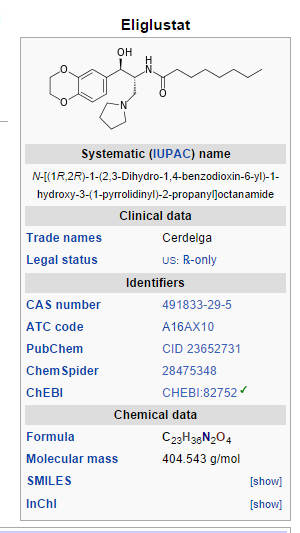
CERDELGA (eliglustat) capsules contain eliglustat tartrate, which is a small molecule inhibitor of glucosylceramide synthase that resembles the ceramide substrate for the enzyme, with the chemical name N-((1R,2R)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1-hydroxy-3-(pyrrolidin-1-yl)propan-2-yl)octanamide (2R,3R)-2,3-dihydroxysuccinate. Its molecular weight is 479.59, and the empirical formula is C23H36N2O4+½(C4H6O6) with the following chemical structure:
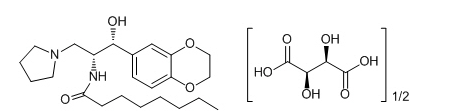
Each capsule of CERDELGA for oral use contains 84 mg of eliglustat, equivalent to 100 mg of eliglustat tartrate (hemitartrate salt). The inactive ingredients are microcrystalline cellulose, lactose monohydrate, hypromellose and glyceryl behenate, gelatin, candurin silver fine, yellow iron oxide, and FD&C blue 2.
Pharmacodynamics
Electrocardiographic Evaluation
- QTc interval prolongation was studied in a double-blind, single dose, placebo- and positive-controlled crossover study in 42 healthy subjects. Concentration-related increases were observed for the placebo-corrected change from baseline in the PR, QRS, and QTc intervals. Based on PK/PD modeling, eliglustat plasma concentrations of 500 ng/mL are predicted to cause mean (upper bound of the 95% one-sided confidence interval) increases in the PR, QRS, and QTcF intervals of 22 (26), 7 (10), and 13 (19) msec, respectively. At the highest geometric mean concentrations of 237 ng/mL following a single supratherapeutic dose tested in the thorough QT study, CERDELGA did not prolong the QT/QTc interval to any clinically relevant extent.
Pharmacokinetics
- At a given dose, the systemic exposure (Cmax and AUC) depends on the CYP2D6 phenotype. In CYP2D6 EMs and IMs, the eliglustat pharmacokinetics is time-dependent and the systemic exposure increases in a more than dose proportional manner. After multiple oral doses of 84 mg twice daily in EMs, eliglustat systemic exposure (AUC0-12) increased up to about 2-fold at steady state compared to after the first dose (AUC0-∞). The pharmacokinetics of eliglustat in CYP2D6 PMs is expected to be linear and time-independent. Compared to EMs, the systemic exposure following 84 mg twice daily at steady state is 7- to 9-fold higher in PMs.
Absorption
- In CYP2D6 EMs, median time to reach maximum plasma concentrations (tmax) occurs at 1.5 to 2 hours following multiple doses of eliglustat 84 mg twice daily. The corresponding mean Cmax values range from 12.1 to 25.0 ng/mL in EMs. The mean AUCtau values range from 76.3 to 143 hr*ng/mL in EMs. The Cmax and AUCtau in one IM subject receiving multiple doses of eliglustat 84 mg two time daily was 44.6 ng/mL and 306 hr*ng/mL, respectively. The oral bioavailability is low in EMs (<5%) following single dose of eliglustat 84 mg due to significant first-pass metabolism.
- In PMs, median tmax occurs at 3 hours following multiple doses of eliglustat 84 mg twice daily. The corresponding mean Cmax and AUCtau values range from 113 to 137 ng/mL and 922 to 1057 hr*ng/mL, respectively.
- Oral dosing of eliglustat 84 mg once daily has not been studied in PMs. The predicted Cmax and AUC0-24hr in PMs using physiologically-based pharmacokinetic (PBPK) model with 84 mg once daily are 75 ng/mL and 956 hr*ng/mL, respectively.
- Administration of eliglustat with a high fat meal resulted in a 15% decrease in Cmax but no change in AUC. Food does not have a clinically relevant effect on eliglustat pharmacokinetics.
Distribution
- Eliglustat is moderately bound to human plasma proteins (76 to 83%). In the blood, it is mainly distributed in plasma and not red blood cells. After intravenous (IV) administration, the volume of distribution of eliglustat was 835 L in CYP2D6 EMs, suggesting wide distribution to tissues (eliglustat is only for oral use).
Metabolism and Elimination
- Eliglustat is extensively metabolized with high clearance, mainly by CYP2D6 and to a lesser extent CYP3A4. Primary metabolic pathways of eliglustat involve sequential oxidation of the octanoyl moiety followed by oxidation of the 2,3-dihydro-1,4-benzodioxane moiety, or a combination of the two pathways, resulting in multiple oxidative metabolites. No active metabolites have been identified.
- After oral administration of 84 mg [14C]-eliglustat, the majority of the administered dose is excreted in urine (41.8%) and feces (51.4%), mainly as metabolites. After 42 mg IV administration in healthy volunteers, mean (CV%) of eliglustat total body clearance was 88 L/h (8.8%) in CYP2D6 EMs (eliglustat is only for oral use). Following multiple oral doses of eliglustat 84 mg twice daily, eliglustat terminal elimination half-life (T1/2) was approximately 6.5 hours in EMs and 8.9 hours in PMs.
Specific Populations
- Based on population PK analysis, there was no effect of mild renal impairment on eliglustat PK. Furthermore, gender, body weight, age, and race had no clinically relevant impact on the pharmacokinetics of eliglustat.
Drug Interactions - Effect of Other Drugs on Eliglustat
- In vitro, eliglustat is metabolized primarily by CYP2D6 and to a lesser extent by CYP3A4. Eliglustat is also a substrate of P-glycoprotein (P-gp).
- Co-administration of eliglustat with CYP2D6 Inhibitors
- Systemic exposure (Cmax and AUCtau) of eliglustat increased 7.0-fold and 8.4-fold, respectively, following co-administration of eliglustat 84 mg twice daily with paroxetine (a strong CYP2D6 inhibitor) 30 mg once daily in EMs (N=30), respectively.
- Simulations using PBPK models suggested that paroxetine may increase the Cmax and AUCtau of eliglustat 2.1- and 2.3-fold in IMs, respectively.
- Compared to paroxetine, the effects of terbinafine (a moderate inhibitor of CYP2D6) on the exposure of eliglustat in EMs or IMs were predicted to be smaller. Simulations using PBPK models suggested that terbinafine may increase the Cmax and AUCtau of eliglustat 3.8- and 4.5-fold in EMs, respectively. Both Cmax and AUCtau increased 1.6-fold in IMs.
Co-administration of Eliglustat with CYP3A Inhibitors
CYP2D6 EMs and IMs:
- Following co-administration of eliglustat 84 mg twice daily with ketoconazole (a strong CYP3A inhibitor) 400 mg once daily, the systemic exposure (Cmax and AUCtau) of eliglustat increased 4.0-fold and 4.4-fold in EMs (N=31).
- Simulations using PBPK models suggested that ketoconazole may increase the Cmax and AUCtau of eliglustat 4.4- and 5.4-fold in IMs, respectively.
- Compared to ketoconazole, the effects of fluconazole (a moderate inhibitor of CYP3A) on the exposure of eliglustat in EMs or IMs were predicted to be smaller. Simulations using PBPK models suggested that fluconazole may increase the Cmax and AUCtau of eliglustat 2.8- and 3.2-fold in EMs, respectively, and 2.5- to 2.9-fold in IMs, respectively.
CYP2D6 PMs:
- The effect of CYP3A inhibitors on the systemic exposure of eliglustat in PMs has not been evaluated in clinical studies. Simulations using PBPK models suggest that ketoconazole may increase the Cmax and AUC0-24h of eliglustat 4.3- and 6.2-fold when co-administered with eliglustat 84 mg once daily in PMs. Simulations using PBPK models suggested that fluconazole may increase the Cmax and AUC0-24h of eliglustat 2.4- and 3.0-fold, respectively, when co-administered with eliglustat 84 mg once daily.
Co-administration of Eliglustat with CYP2D6 and CYP3A inhibitors
- Simulations using PBPK models suggested that concomitant use of eliglustat 84 mg twice daily with paroxetine and ketoconazole may increase the Cmax and AUCtau of eliglustat 16.7- and 24.2-fold in EMs, respectively. The predicted Cmax and AUCtau of eliglustat increased 7.5- to 9.8-fold in IMs, respectively.
- Simulations using PBPK models suggested that concomitant use of eliglustat 84 mg twice daily with terbinafine and fluconazole may increase the Cmax and AUCtau of eliglustat 10.2- and 13.6-fold in EMs. The predicted Cmax and AUCtau of eliglustat increased 4.2- to 5.0-fold in IMs, respectively.
Effect of CYP3A inducers on Eliglustat PK
- Systemic exposures (Cmax and AUCtau) of eliglustat decreased by approximately 90% in EMs and IMs, following co-administration of eliglustat 127 mg twice daily with rifampin (a strong CYP3A inducer) 600 mg PO once daily. The only approved dose of eliglustat is 84 mg. Systemic exposures of eliglustat decreased by approximately 95% following co-administration of eliglustat 84 mg twice daily with rifampin 600 mg PO once daily in PMs.
Effect of OATP (organic anion transporting polypeptide) Inhibitors on Eliglustat PK
- Systemic exposures of eliglustat were similar with or without co-administration of single 600 mg IV dose of rifampin (a potent OATP inhibitor) regardless of subjects' CYP2D6 phenotypes.
Effect of P-gp Inhibitors on Eliglustat PK
- The effect of P-gp inhibitors on the systemic exposure of eliglustat has not been studied clinically.
Effect of Gastric pH-Modifying Agents on Eliglustat PK
- Gastric pH-modifying agents (Maalox®, Tums®, Protonix®) did not have a clinically relevant effect on eliglustat exposure.
Drug Interactions - Effect of eliglustat on the PK of Other Drugs
- Eliglustat is an inhibitor of P-gp and CYP2D6.
- Following multiple doses of eliglustat 127 mg twice daily, systemic exposures (Cmax and AUC) to metoprolol (a CYP2D6 substrate) increased compared to metoprolol administration alone. Mean Cmax and AUC increased by 1.7- and 2.3-fold, respectively, in EMs and by 1.2- and 1.6-fold, respectively in IMs. The only approved dose of eliglustat is 84 mg.
- Following multiple doses of eliglustat 127 mg twice daily in EMs and IMs or 84 mg twice daily in PMs, systemic exposures (Cmax and AUC) to digoxin (a P-gp substrate, with narrow therapeutic index) increased compared to digoxin administration alone. Mean Cmax and AUC increased by 1.7- and 1.5-fold, respectively. The only approved dose of eliglustat is 84 mg.
- In vitro, eliglustat is a weak inhibitor of CYP3A. Repeated doses of eliglustat 84 mg twice daily did not change the exposures to norethindrone (1.0 mg) and ethinyl estradiol (0.035 mg). Therefore, eliglustat is not expected to impact the efficacy or safety of oral contraceptives containing norethindrone and ethinyl estradiol.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Carcinogenic potential of eliglustat was assessed in 2-year carcinogenicity studies in rats and mice. In Sprague-Dawley rats, eliglustat was administered by oral gavage at doses up to 75 mg/kg/day in males (about 3.6 times the recommended human daily dose of 84 mg twice daily, based on body surface area) and 50 mg/kg/day in females (about 2.4 times the recommended human daily dose based on body surface area). In CD-1 mice, eliglustat was administered to males and females at up to 75 mg/kg/day (about 1.8 times the recommended human daily dose based on body surface area) via dietary admixture. Eliglustat did not produce any treatment-related neoplasms in rats or mice.
Mutagenesis
- Eliglustat was negative in the Ames test, chromosome aberration test in human peripheral blood lymphocytes, mouse lymphoma gene mutation assay and in vivo oral mouse micronucleus test.
Impairment of Fertility
- In a fertility and early embryonic development study in rats, eliglustat increased pre-implantation loss at 30 (about 1.5 times the recommended human oral dose based on body surface area) and 100 mg/kg/day (about 5 times the recommended human oral dose based on body surface area).
- In mature male rats, eliglustat showed reversible adverse effects on sperm morphology, testes (germ cell necrosis), and sloughed cells in the epididymis at 200 mg/kg/day (about 10 times the recommended human oral dose based on body surface area). Similar effects on sperm were not seen in mature Cynomolgus monkeys at 72 mg/kg/day (about 7 times the recommended human oral dose based on body surface area).
Clinical Studies
- The efficacy of eliglustat was evaluated in three clinical trials in patients with Gaucher disease type 1.
Eliglustat in Treatment-Naïve GD1 Patients – Trial 1
- Trial 1 was a randomized, double-blind, placebo-controlled, multicenter clinical study evaluating the efficacy and safety of eliglustat in 40 treatment-naïve GD1 patients 16 years of age or older (median age 30.4 years) with pre-existing splenomegaly and hematological abnormalities. Patients were required to have received no treatment with substrate reduction therapy within 6 months or ERT within 9 months prior to randomization; all but 5 patients in the study had no prior therapy. Patients were stratified according to baseline spleen volume (≤ 20 or > 20 multiples of normal [MN]) and randomized in a 1:1 ratio to receive eliglustat or placebo for the duration of the 9-month blinded primary analysis period. The eliglustat treatment group was comprised of IM (5%), EM (90%) and URM (5%) patients. Patients randomized to eliglustat treatment received a starting dose of 42 mg twice daily, with a dose increase to 84 mg twice daily possible at Week 4 based on the plasma trough concentration at Week 2. The majority of patients (17 [85%]) received a dose escalation to 84 mg twice daily at Week 4, and 3 (15%) continued to receive 42 mg twice daily for the duration of the 9-month blinded primary analysis period.
- The primary endpoint was the percentage change in spleen volume (in MN) from baseline to 9 months as compared to placebo. Secondary endpoints were absolute change in hemoglobin level, percentage change in liver volume (in MN), and percentage change in platelet count from baseline to 9 months compared to placebo.
- At baseline, mean spleen volumes were 12.5 and 13.9 MN in the placebo and eliglustat groups, respectively, and mean liver volumes were 1.4 MN for both groups. Mean hemoglobin levels were 12.8 and 12.1 g/dL, and platelet counts were 78.5 and 75.1 x 109/L, respectively.
- During the 9-month primary analysis period, eliglustat demonstrated statistically significant improvements in all primary and secondary endpoints compared to placebo, as shown in Table 6.
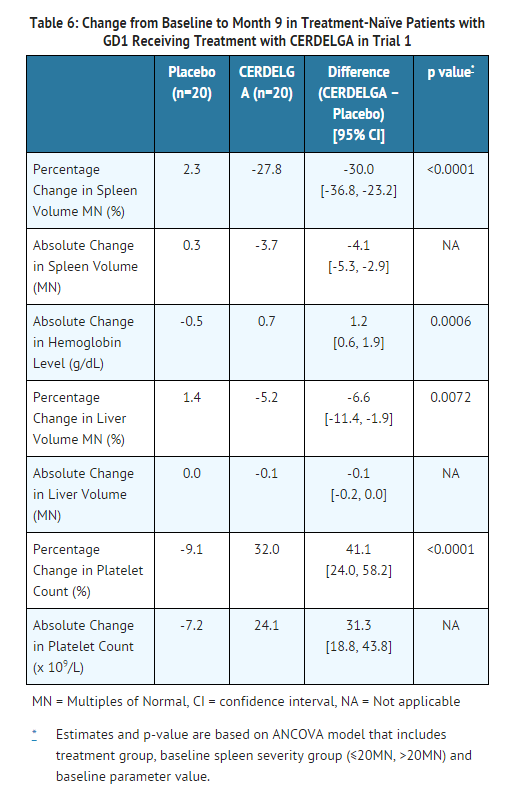
- In an uncontrolled study of treatment naïve GD1 patients, improvements in spleen and liver volume, hemoglobin level, and platelet count continued through the 4 year treatment period.
Patients Switching from Enzyme Replacement Therapy to Eliglustat – Trial 2
- Trial 2 was a randomized, open-label, active-controlled, non-inferiority, multicenter clinical study evaluating the efficacy and safety of eliglustat compared with imiglucerase in 159 treated GD1 patients (median age 37.4 years) previously treated with enzyme replacement therapy (≥3 years of enzyme replacement therapy, dosed at 30-130 U/kg/month in at least 6 of the prior 9 months) who met pre-specified therapeutic goals at baseline. Pre-specified baseline therapeutic goals included: no bone crisis and free of symptomatic bone disease within the last year; mean hemoglobin level of ≥ 11 g/dL in females and ≥ 12 g/dL in males; mean platelet count ≥ 100,000/mm3; spleen volume < 10 times normal and liver volume < 1.5 times normal.
- Patients were randomized 2:1 to receive eliglustat or imiglucerase for the duration of the 12-month primary analysis period. Seventy-five percent of patients randomized to eliglustat were previously treated with imiglucerase; 21% with velaglucerase alfa and 4% were unreported. Patients randomized to eliglustat treatment received a starting dose of 42 mg twice daily, with dose increases to 84 mg twice daily and 127 mg twice daily possible at Weeks 4 and 8 based on plasma trough concentrations of eliglustat at Weeks 2 and 6, respectively. The percentage of patients receiving the 3 possible eliglustat doses was: 42 mg twice daily (20%), 84 mg twice daily (32%) and 127 mg twice daily (48%). The eliglustat treatment group was comprised of PM (4%), IM (10%), EM (80%) and URM (4%) patients.
- At baseline, mean spleen volumes were 2.6 and 3.2 MN in the imiglucerase and eliglustat groups, respectively, and liver volumes were 0.9 MN in both groups. Mean hemoglobin levels were 13.8 and 13.6 g/dL, and platelet counts were 192 and 207 x 109/L, respectively.
- The primary composite endpoint required stability in all four component domains (hemoglobin level, platelet count, liver volume, and spleen volume) based on changes between baseline and 12 months. Stability was defined by the following pre-specified thresholds of change: hemoglobin level <1.5 g/dL decrease, platelet count < 25% decrease, liver volume <20% increase and spleen volume <25% increase. The percentages of patients meeting the criteria for stability in the individual components of the composite endpoint were assessed as secondary efficacy endpoints.
- Eliglustat met the criteria to be declared non-inferior to imiglucerase in maintaining patient stability. After 12 months of treatment, the percentage of patients meeting the primary composite endpoint was 84.8% for the eliglustat group compared to 93.6% for the imiglucerase group. The lower bound of the 95% CI of the 8.8% difference, -17.6%, was within the pre-specified non-inferiority margin of -25%. At Month 12, the percentages of CERDELGA and imiglucerase patients respectively, who met stability criteria for the individual components of the composite endpoint were: hemoglobin level, 94.9% and 100%; platelet count, 92.9% and 100%; spleen volume, 95.8% and 100%; and liver volume, 96.0% and 93.6%. Of the patients who did not meet stability criteria for the individual components, 12 of 15 CERDELGA patients and 3 of 3 imiglucerase patients remained within therapeutic goals for GD1.
- Mean changes from baseline in the hematological and visceral parameters through 12 months of treatment are shown in Table 7. There were no clinically meaningful differences between groups for any of the four parameters.
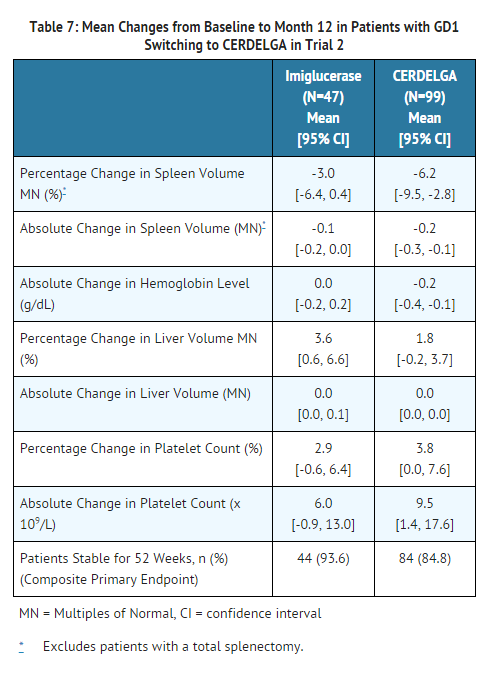
How Supplied
- Eliglustat is supplied as 84 mg hard gelatin capsules, with a pearl blue-green opaque cap and pearl white opaque body imprinted with "GZ02" in black.
- Eliglustat 84 mg capsules are supplied as:
NDC-58468-0220-1 – Carton containing 4 packs of capsules (56 capsules total). Each pack is composed of 1 blister card of 14 capsules and a cardboard wallet.
NDC-58468-0220-2 – Carton containing 1 pack of capsules (14 capsules total). Each pack is comprised of 1 blister card of 14 capsules and a cardboard wallet.
Storage
- Store at 68 °F - 77 °F (20 °C - 25 °C) with excursions permitted between 59 °F and 86 °F (15 °C to 30 °C) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Eliglustat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eliglustat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Drug Interactions
- Advise patients to discuss all the medications they are taking, including any herbal supplements or vitamins with their healthcare provider.
ECG Changes and Potential for Cardiac Arrhythmias
- Advise patients to inform their healthcare provider of the following: history of congestive heart failure; recent acute myocardial infarction; bradycardia; heart block; ventricular arrhythmia; and long QT syndrome.
- Advise patients to inform their healthcare provider if they develop new symptoms such as palpitations, fainting, and dizziness.
Administration Instructions
Advise patients:
- Swallow capsules whole, preferably with water, and do not crush, dissolve, or open the capsules.
- CERDELGA can be taken with or without food.
- If a dose of CERDELGA is missed, take the prescribed dose at the next scheduled time; do not double the next dose.
- Avoid consumption of grapefruit or its juice.
- For patients currently treated with imiglucerase, velaglucerase alfa, or taliglucerase alfa, CERDELGA may be administered 24 hours after the last dose of the previous enzyme replacement therapy (ERT).
Precautions with Alcohol
- Alcohol-Eliglustat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CERDELGA
Look-Alike Drug Names
There is limited information regarding Eliglustat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Eliglustat
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Eliglustat |Label Name=Eliglustat image.jpg
}}
{{#subobject:
|Label Page=Eliglustat |Label Name=Eliglustat ingredients and appearance.png
}}
{{#subobject:
|Label Page=Eliglustat |Label Name=Eliglustat medication guide.png
}}