Eculizumab: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=monoclonal antibody | |drugClass=monoclonal antibody | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and for patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy | |indication=patients with [[paroxysmal nocturnal hemoglobinuria]] (PNH) to reduce [[hemolysis]] and for patients with atypical [[hemolytic uremic syndrome]] (aHUS) to inhibit complement-mediated [[thrombotic microangiopathy]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=hypertension, diarrhea, nausea, vomiting, anemia, backache, headache, insomnia, nasal congestion, nasopharyngitis, respiratory tract infection and fever | |adverseReactions=[[hypertension]], [[diarrhea]], [[nausea]], [[vomiting]], [[anemia]], [[backache]], [[headache]], [[insomnia]], [[nasal congestion]], [[nasopharyngitis]], [[respiratory tract infection]] and [[fever]] | ||
|blackBoxWarningTitle=WARNING: SERIOUS MENINGOCOCCAL INFECTIONS | |blackBoxWarningTitle=WARNING: SERIOUS MENINGOCOCCAL INFECTIONS | ||
|blackBoxWarningBody=Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. | |blackBoxWarningBody=Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. | ||
* Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients with complement deficiencies. | * Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients with complement deficiencies. | ||
* Immunize patients with a meningococcal vaccine at least 2 weeks prior to administering the first dose of | * Immunize patients with a meningococcal vaccine at least 2 weeks prior to administering the first dose of eculizumab, unless the risks of delaying eculizumab therapy outweigh the risk of developing a meningococcal infection. | ||
* Monitor patients for early signs of meningococcal infections and evaluate immediately if infection is suspected. | * Monitor patients for early signs of meningococcal infections and evaluate immediately if infection is suspected. | ||
Eculizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the | Eculizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the eculizumab REMS, prescribers must enroll in the program | ||
|fdaLIADAdult=====Paroxysmal Nocturnal Hemoglobinuria (PNH)==== | |fdaLIADAdult=====Paroxysmal Nocturnal Hemoglobinuria (PNH)==== | ||
Eculizumab is indicated for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis. | Eculizumab is indicated for the treatment of patients with [[paroxysmal nocturnal hemoglobinuria]] (PNH) to reduce [[hemolysis]]. | ||
Dosage: | Dosage: | ||
* 600 mg weekly for the first 4 weeks, followed by | * 600 mg weekly for the first 4 weeks, followed by | ||
| Line 22: | Line 22: | ||
====Atypical Hemolytic Uremic Syndrome (aHUS)==== | ====Atypical Hemolytic Uremic Syndrome (aHUS)==== | ||
Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. | Eculizumab is indicated for the treatment of patients with atypical [[hemolytic uremic syndrome]] (aHUS) to inhibit complement-mediated thrombotic microangiopathy. | ||
Dosage: | Dosage: | ||
* 900 mg weekly for the first 4 weeks, followed by | * 900 mg weekly for the first 4 weeks, followed by | ||
| Line 30: | Line 30: | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Eculizumab in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Eculizumab in adult patients. | ||
|fdaLIADPed=====Atypical Hemolytic Uremic Syndrome (aHUS)==== | |fdaLIADPed=====Atypical Hemolytic Uremic Syndrome (aHUS)==== | ||
* Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. | * Eculizumab is indicated for the treatment of patients with atypical [[hemolytic uremic syndrome]] (aHUS) to inhibit complement-mediated [[thrombotic microangiopathy]]. | ||
* For patients less than 18 years of age, administer eculizumab based upon body weight, according to the following schedule: | * For patients less than 18 years of age, administer eculizumab based upon body weight, according to the following schedule: | ||
[[File:Eculizumab dosage.png|thumb|none|500px]] | [[File:Eculizumab dosage.png|thumb|none|500px]] | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Eculizumab in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Eculizumab in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Eculizumab in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Eculizumab in pediatric patients. | ||
|contraindications=* Patients with unresolved serious Neisseria meningitidis infection. | |contraindications=* Patients with unresolved serious [[Neisseria meningitidis]] infection. | ||
* Patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying | * Patients who are not currently vaccinated against [[Neisseria meningitidis]], unless the risks of delaying eculizumab treatment outweigh the risks of developing a meningococcal infection | ||
|warnings=====Serious Meningococcal Infections==== | |warnings=====Serious Meningococcal Infections==== | ||
* The use of | * The use of eculizumab increases a patient's susceptibility to serious meningococcal infections (septicemia and/or meningitis). Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. | ||
* Administer a polyvalent meningococcal vaccine according to the most current Advisory Committee on Immunization Practices (ACIP) recommendations for patients with complement deficiencies. Revaccinate patients in accordance with ACIP recommendations, considering the duration of | * Administer a polyvalent meningococcal vaccine according to the most current Advisory Committee on Immunization Practices (ACIP) recommendations for patients with complement deficiencies. Revaccinate patients in accordance with ACIP recommendations, considering the duration of eculizumab therapy. | ||
* Immunize patients without a history of meningococcal vaccination at least 2 weeks prior to receiving the first dose of | * Immunize patients without a history of meningococcal vaccination at least 2 weeks prior to receiving the first dose of eculizumab. If urgent eculizumab therapy is indicated in an unvaccinated patient, administer the meningococcal vaccine as soon as possible. In prospective clinical studies, 75/100 patients with aHUS were treated with eculizumab less than 2 weeks after meningococcal vaccination and 64 of these 75 patients received antibiotics for prophylaxis of meningococcal infection until at least 2 weeks after meningococcal vaccination. The benefits and risks of antibiotic prophylaxis for prevention of meningococcal infections in patients receiving eculizumab have not been established. | ||
* Vaccination reduces, but does not eliminate, the risk of meningococcal infections. In clinical studies, 2 out of 196 PNH patients developed serious meningococcal infections while receiving treatment with | * Vaccination reduces, but does not eliminate, the risk of meningococcal infections. In clinical studies, 2 out of 196 PNH patients developed serious meningococcal infections while receiving treatment with eculizumab; both had been vaccinated. In clinical studies among non-PNH patients, meningococcal meningitis occurred in one unvaccinated patient. In addition, 3 out of 130 previously vaccinated patients with aHUS developed meningococcal infections while receiving treatment with eculizumab. | ||
* Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if an infection is suspected. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Discontinue | * Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if an infection is suspected. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Discontinue eculizumab in patients who are undergoing treatment for serious meningococcal infections. | ||
==== | ====Eculizumab REMS==== | ||
* Because of the risk of meningococcal infections, | * Because of the risk of meningococcal infections, eculizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the eculizumab REMS, prescribers must enroll in the program. | ||
* Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with a meningococcal vaccine. | * Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with a meningococcal vaccine. | ||
====Other Infections==== | ====Other Infections==== | ||
* | * eculizumab blocks terminal complement activation; therefore patients may have increased susceptibility to infections, especially with encapsulated bacteria. Additionally, Aspergillus infections have occurred in immunocompromised and neutropenic patients. Children treated with eculizumab may be at increased risk of developing serious infections due to Streptococcuspneumoniae and Haemophilus influenza type b (Hib). Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenza type b (Hib) infections according to ACIP guidelines. Use caution when administering eculizumab to patients with any systemic infection. | ||
====Monitoring Disease Manifestations After | ====Monitoring Disease Manifestations After eculizumab Discontinuation==== | ||
=====Treatment Discontinuation for PNH===== | =====Treatment Discontinuation for PNH===== | ||
* Monitor patients after discontinuing | * Monitor patients after discontinuing eculizumab for at least 8 weeks to detect hemolysis. | ||
=====Treatment Discontinuation for aHUS===== | =====Treatment Discontinuation for aHUS===== | ||
* After discontinuing | * After discontinuing eculizumab, monitor patients with aHUS for signs and symptoms of thrombotic microangiopathy (TMA) complications for at least 12 weeks. In aHUS clinical trials, 18 patients (5 in the prospective studies) discontinued eculizumab treatment. TMA complications occurred following a missed dose in 5 patients, and eculizumab was reinitiated in 4 of these 5 patients. | ||
Clinical signs and symptoms of TMA include changes in mental status, seizures, angina, dyspnea, or thrombosis. In addition, the following changes in laboratory parameters may identify a TMA complication: occurrence of two, or repeated measurement of any one of the following: a decrease in platelet count by 25% or more compared to baseline or the peak platelet count during | Clinical signs and symptoms of TMA include changes in mental status, seizures, angina, dyspnea, or thrombosis. In addition, the following changes in laboratory parameters may identify a TMA complication: occurrence of two, or repeated measurement of any one of the following: a decrease in platelet count by 25% or more compared to baseline or the peak platelet count during eculizumab treatment; an increase in serum creatinine by 25% or more compared to baseline or nadir during eculizumab treatment; or, an increase in serum LDH by 25% or more over baseline or nadir during eculizumab treatment. | ||
If TMA complications occur after | If TMA complications occur after eculizumab discontinuation, consider reinstitution of eculizumab treatment, plasma therapy [plasmapheresis, plasma exchange, or fresh frozen plasma infusion (PE/PI)], or appropriate organ-specific supportive measures. | ||
====Thrombosis Prevention and Management==== | ====Thrombosis Prevention and Management==== | ||
* The effect of withdrawal of anticoagulant therapy during | * The effect of withdrawal of anticoagulant therapy during eculizumab treatment has not been established. Therefore, treatment with eculizumab should not alter anticoagulant management. | ||
====Infusion Reactions==== | ====Infusion Reactions==== | ||
* As with all protein products, administration of | * As with all protein products, administration of eculizumab may result in infusion reactions, including anaphylaxis or other hypersensitivity reactions. In clinical trials, no patients experienced an infusion reaction which required discontinuation of eculizumab. Interrupt eculizumab infusion and institute appropriate supportive measures if signs of cardiovascular instability or respiratory compromise occur. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
Meningococcal infections are the most important adverse reactions experienced by patients receiving | Meningococcal infections are the most important adverse reactions experienced by patients receiving eculizumab. In PNH clinical studies, two patients experienced meningococcal sepsis. Both patients had previously received a meningococcal vaccine. In clinical studies among patients without PNH, meningococcal meningitis occurred in one unvaccinated patient. Meningococcal sepsis occurred in one previously vaccinated patient enrolled in the retrospective aHUS study during the post-study follow-up period. | ||
====PNH==== | ====PNH==== | ||
The data described below reflect exposure to | The data described below reflect exposure to eculizumab in 196 adult patients with PNH, age 18-85, of whom 55% were female. All had signs or symptoms of intravascular hemolysis. eculizumab was studied in a placebo-controlled clinical study (in which 43 patients received eculizumab and 44, placebo); a single arm clinical study and a long term extension study. 182 patients were exposed for greater than one year. All patients received the recommended eculizumab dose regimen. | ||
[[File:Eculizumab adverse reactions PNH.png|thumb|none|500px]] | [[File:Eculizumab adverse reactions PNH.png|thumb|none|500px]] | ||
In the placebo-controlled clinical study, serious adverse reactions occurred among 4 (9%) patients receiving | In the placebo-controlled clinical study, serious adverse reactions occurred among 4 (9%) patients receiving eculizumab and 9 (21%) patients receiving placebo. The serious reactions included infections and progression of PNH. No deaths occurred in the study and no patients receiving eculizumab experienced a thrombotic event; one thrombotic event occurred in a patient receiving placebo. | ||
Among 193 patients with PNH treated with | Among 193 patients with PNH treated with eculizumab in the single arm, clinical study or the follow-up study, the adverse reactions were similar to those reported in the placebo-controlled clinical study. Serious adverse reactions occurred among 16% of the patients in these studies. The most common serious adverse reactions were: viral infection (2%), headache (2%), anemia (2%), and pyrexia (2%). | ||
====aHUS==== | ====aHUS==== | ||
The safety of | The safety of eculizumab therapy in patients with aHUS was evaluated in four prospective, single-arm studies, three in adult and adolescent patients (aHUS Studies 1, 2, and 4), one in pediatric and adolescent patients (aHUS Study 5) and one retrospective study (aHUS Study 3). | ||
The data described below were derived from 78 adult and adolescent patients with aHUS enrolled in aHUS Study 1, aHUS Study 2, and aHUS Study 4. All patients received the recommended dosage of | The data described below were derived from 78 adult and adolescent patients with aHUS enrolled in aHUS Study 1, aHUS Study 2, and aHUS Study 4. All patients received the recommended dosage of eculizumab. Median exposure was 67 weeks (range: 2-145 weeks). Table 5 summarizes all adverse events reported in at least 10% of patients in aHUS Studies 1, 2, and 4 combined. | ||
[[File:Eculizumab adverse reactions aHUS.png|thumb|none|600px]] | [[File:Eculizumab adverse reactions aHUS.png|thumb|none|600px]] | ||
In aHUS Studies 1, 2, and 4 combined, 60% (47/78) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were infections (24%), hypertension (5%), chronic renal failure (5%), and renal impairment (5%). Five patients discontinued | In aHUS Studies 1, 2, and 4 combined, 60% (47/78) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were infections (24%), hypertension (5%), chronic renal failure (5%), and renal impairment (5%). Five patients discontinued eculizumab due to adverse events; three due to worsening renal function, one due to new diagnosis of Systemic Lupus Erythematosus, and one due to meningoccal meningitis. | ||
aHUS Study 5 included 22 pediatric and adolescent patients, of which 18 patients were less than 12 years of age. All patients received the recommended dosage of | aHUS Study 5 included 22 pediatric and adolescent patients, of which 18 patients were less than 12 years of age. All patients received the recommended dosage of eculizumab. Median exposure was 44 weeks (range: 1 dose-87 weeks). | ||
[[File:Eculizumab adverse reactions aHUS study 5.png|thumb|none|600px]] | [[File:Eculizumab adverse reactions aHUS study 5.png|thumb|none|600px]] | ||
In aHUS Study 5, 59% (13/22) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were hypertension (9%), viral gastroenteritis (9%), pyrexia (9%), and upper respiratory infection (9%). One patient discontinued | In aHUS Study 5, 59% (13/22) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were hypertension (9%), viral gastroenteritis (9%), pyrexia (9%), and upper respiratory infection (9%). One patient discontinued eculizumab due to an adverse event (severe agitation). | ||
Analysis of retrospectively collected adverse event data from pediatric and adult patients enrolled in aHUS Study 3 (N=30) revealed a safety profile that was similar to that which was observed in the two prospective studies. aHUS Study 3 included 19 pediatric patients less than 18 years of age. Overall, the safety of | Analysis of retrospectively collected adverse event data from pediatric and adult patients enrolled in aHUS Study 3 (N=30) revealed a safety profile that was similar to that which was observed in the two prospective studies. aHUS Study 3 included 19 pediatric patients less than 18 years of age. Overall, the safety of eculizumab in pediatric patients with aHUS enrolled in Study 3 appeared similar to that observed in adult patients. The most common (≥15%) adverse events occurring in pediatric patients are presented in Table 7. | ||
[[File:Eculizumab adverse reactions pediatric patients.png|thumb|none|600px]] | [[File:Eculizumab adverse reactions pediatric patients.png|thumb|none|600px]] | ||
====Immunogenicity==== | ====Immunogenicity==== | ||
As with all proteins, there is a potential for immunogenicity with eculizumab. The immunogenicity of | As with all proteins, there is a potential for immunogenicity with eculizumab. The immunogenicity of eculizumab has been evaluated using two different immunoassays for the detection of anti-eculizumab antibodies: a direct enzyme-linked immunosorbent assay (ELISA) using the Fab fragment of eculizumab as target was used for the PNH indication; and an electro-chemiluminescence (ECL) bridging assay using the eculizumab whole molecule as target was used for the aHUS indication, as well as for additional patients with PNH. In the PNH population, antibodies to eculizumab were detected in 3/196 (2%) patients with PNH treated with eculizumab using the ELISA assay and in 5/161 (3%) patients treated with eculizumab using the ECL assay. In patients with aHUS treated with eculizumab, antibodies to eculizumab were detected in 3/100 (3%) using the ECL assay. An ECL based neutralizing HAHA assay with a low sensitivity of 2 mcg/mL was performed to detect neutralizing antibodies for the 3 patients with aHUS and also for the 5 patients with PNH with positive samples using the ECL assay. 2/161 patients in the PNH group (1.2%) and 1/100 patients in the aHUS group (1%) had low positive values for neutralizing antibodies. No apparent correlation of antibody development to clinical response was observed in either indication. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to eculizumab in an ELISA-based assay and/or an ECL-based assay and are highly dependent on the sensitivity and specificity of the assay used. Additionally, the observed incidence of antibody positivity in the assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to eculizumab with the incidence of antibodies to other products may be misleading. | ||
|postmarketing=The following adverse reactions have been identified during post-approval use of | |postmarketing=The following adverse reactions have been identified during post-approval use of eculizumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to eculizumab exposure. | ||
Cases of serious or fatal meningococcal infections have been reported. | Cases of serious or fatal meningococcal infections have been reported. | ||
|drugInteractions=Drug interaction studies have not been performed with | |drugInteractions=Drug interaction studies have not been performed with eculizumab. | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA= | |useInPregnancyFDA=There are no adequate and well-controlled studies of eculizumab in pregnant women. eculizumab, a recombinant IgG molecule (humanized anti-C5 antibody), is expected to cross the placenta. Animal studies using a mouse analogue of the eculizumab molecule (murine anti-C5 antibody) showed increased rates of developmental abnormalities and an increased rate of dead and moribund offspring at doses 2-8 times the human dose. eculizumab should be used during pregnancy only if the potential benefit justifies the potential risk to the [[fetus]]. | ||
There are no adequate and well-controlled studies of | |||
====Animal Data==== | ====Animal Data==== | ||
Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 2-4 times (low dose) and 4-8 times (high dose) the recommended human | Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 2-4 times (low dose) and 4-8 times (high dose) the recommended human eculizumab dose, based on a body weight comparison. When animal exposure to the antibody occurred in the time period from before mating until early gestation, no decrease in fertility or reproductive performance was observed. When maternal exposure to the antibody occurred during organogenesis, two cases of retinal dysplasia and one case of umbilical hernia were observed among 230 offspring born to mothers exposed to the higher antibody dose; however, the exposure did not increase fetal loss or neonatal death. When maternal exposure to the antibody occurred in the time period from implantation through weaning, a higher number of male offspring became moribund or died (1/25 controls, 2/25 low dose group, 5/25 high dose group). Surviving offspring had normal development and reproductive function. | ||
|useInNursing=It is not known whether | |useInNursing=It is not known whether eculizumab is excreted into human milk. IgG is excreted in human milk, so it is expected that eculizumab will be present in human milk. However, published data suggest that antibodies in human milk do not enter the neonatal and infant circulation in substantial amounts. Caution should be exercised when eculizumab is administered to a nursing woman. The unknown risks to the infant from gastrointestinal or limited systemic exposure to eculizumab should be weighed against the known benefits of human milk feeding. | ||
|useInPed=The safety and effectiveness of | |useInPed=The safety and effectiveness of eculizumab for the treatment of PNH in pediatric patients below the age of 18 years have not been established. | ||
Four clinical studies assessing the safety and effectiveness of | Four clinical studies assessing the safety and effectiveness of eculizumab for the treatment of aHUS included a total of 47 pediatric patients (ages 2 months to 17 years). The safety and effectiveness of eculizumab for the treatment of aHUS appear similar in pediatric and adult patients. | ||
Administer vaccinations for the prevention of infection due to Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to ACIP guidelines. | Administer vaccinations for the prevention of infection due to [[Neisseria meningitidis]], [[Streptococcus pneumoniae]] and [[Haemophilus influenza]] type b (Hib) according to ACIP guidelines. | ||
|useInGeri=Nineteen patients 65 years of age or older (15 with PNH and 4 with aHUS) were treated with | |useInGeri=Nineteen patients 65 years of age or older (15 with PNH and 4 with aHUS) were treated with eculizumab. Although there were no apparent age-related differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients. | ||
|useInReproPotential=Effects of eculizumab upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 4-8 times the equivalent of the clinical dose of | |useInReproPotential=Effects of eculizumab upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 4-8 times the equivalent of the clinical dose of eculizumab had no adverse effects on mating or fertility. | ||
|administration=Intravenous | |administration=Intravenous | ||
|overdose=No cases of | |overdose=No cases of eculizumab overdose have been reported during clinical studies. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 137: | Line 136: | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = eculizumab | ||
| Drugs.com = {{drugs.com|monograph|eculizumab}} | | Drugs.com = {{drugs.com|monograph|eculizumab}} | ||
| licence_EU = | | licence_EU = eculizumab | ||
| licence_US = Eculizumab | | licence_US = Eculizumab | ||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| Line 177: | Line 176: | ||
| molecular_weight = 148 [[Atomic mass unit|kDa]] | | molecular_weight = 148 [[Atomic mass unit|kDa]] | ||
}} | }} | ||
|mechAction=Eculizumab, the active ingredient in | |mechAction=Eculizumab, the active ingredient in eculizumab, is a monoclonal antibody that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9. eculizumab inhibits terminal complement mediated intravascular hemolysis in PNH patients and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS. | ||
A genetic mutation in patients with PNH leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors, rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia), and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots. | A genetic mutation in patients with PNH leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors, rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia), and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots. | ||
| Line 183: | Line 182: | ||
In aHUS, impairment in the regulation of complement activity leads to uncontrolled terminal complement activation, resulting in platelet activation, endothelial cell damage and thrombotic microangiopathy. | In aHUS, impairment in the regulation of complement activity leads to uncontrolled terminal complement activation, resulting in platelet activation, endothelial cell damage and thrombotic microangiopathy. | ||
|structure=Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa. | |structure=Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa. | ||
|PD=In the PNH placebo-controlled clinical study, | |PD=In the PNH placebo-controlled clinical study, eculizumab when administered as recommended reduced hemolysis as shown by the reduction of serum LDH levels from 2200 ± 1034 U/L (mean ± SD) at baseline to 700 ± 388 U/L by week one and maintained the effect through the end of the study at week 26 (327 ± 433 U/L). In the single arm clinical study, eculizumab maintained this effect through 52 weeks. | ||
|PK=A population PK analysis with a standard 1-compartmental model was conducted on the multiple dose PK data from 40 PNH patients receiving the recommended | |PK=A population PK analysis with a standard 1-compartmental model was conducted on the multiple dose PK data from 40 PNH patients receiving the recommended eculizumab regimen. In this model, the clearance of eculizumab for a typical PNH patient weighing 70 kg was 22 mL/hr and the volume of distribution was 7.7 L. The half-life was 272 ± 82 hrs (mean ± SD). The mean observed peak and trough serum concentrations of eculizumab by week 26 were 194 ± 76 mcg/mL and 97 ± 60 mcg/mL, respectively. | ||
A second population PK analysis with a standard 1 compartmental model was conducted on the multiple dose PK data from 57 aHUS patients receiving the recommended | A second population PK analysis with a standard 1 compartmental model was conducted on the multiple dose PK data from 57 aHUS patients receiving the recommended eculizumab regimen in studies 1, 2 and 3. In this model, the clearance of eculizumab for a typical aHUS patient weighing 70 kg was 14.6 mL/hr and the volume of distribution was 6.14 L. The elimination half-life was 291 h (approximately 12.1 days). | ||
The clearance and half-life of eculizumab were also evaluated during plasma exchange interventions. Plasma exchange increased the clearance of eculizumab to 3660 mL/hr and reduced the half-life to 1.26 hours. Supplemental dosing is recommended when | The clearance and half-life of eculizumab were also evaluated during plasma exchange interventions. Plasma exchange increased the clearance of eculizumab to 3660 mL/hr and reduced the half-life to 1.26 hours. Supplemental dosing is recommended when eculizumab is administered to aHUS patients receiving plasma infusion or exchange. | ||
Dedicated studies have not been conducted to evaluate the PK of | Dedicated studies have not been conducted to evaluate the PK of eculizumab in special patient populations identified by gender, race, age (geriatric), or the presence of renal or hepatic impairment. Pediatric and adolescent patients (less than 18 years of age) and patients with renal impairment were included in the aHUS clinical studies. Population PK analysis showed age, gender, race, and renal function do not influence the PK of eculizumab. | ||
|nonClinToxic=====Carcinogenesis, Mutagenesis==== | |nonClinToxic=====Carcinogenesis, Mutagenesis==== | ||
* Long-term animal carcinogenicity studies of eculizumab have not been conducted. | * Long-term animal carcinogenicity studies of eculizumab have not been conducted. | ||
* Genotoxicity studies have not been conducted with eculizumab. | * Genotoxicity studies have not been conducted with eculizumab. | ||
|clinicalStudies=====PNH==== | |clinicalStudies=====PNH==== | ||
The safety and efficacy of | The safety and efficacy of eculizumab in PNH patients with [[hemolysis]] were assessed in a randomized, double-blind, placebo-controlled 26 week study (Study 1); PNH patients were also treated with eculizumab in a single arm 52 week study (Study 2); and in a long term extension study. Patients received meningococcal vaccination prior to receipt of eculizumab. In all studies, the dose of eculizumab was 600 mg study drug every 7 ± 2 days for 4 weeks, followed by 900 mg 7 ± 2 days later, then 900 mg every 14 ± 2 days for the study duration. eculizumab was administered as an intravenous infusion over 25 - 45 minutes. | ||
======Study 1====== | ======Study 1====== | ||
PNH patients with at least four transfusions in the prior 12 months, flow cytometric confirmation of at least 10% PNH cells and platelet counts of at least 100,000/microliter were randomized to either | PNH patients with at least four transfusions in the prior 12 months, flow cytometric confirmation of at least 10% PNH cells and platelet counts of at least 100,000/microliter were randomized to either eculizumab (n = 43) or placebo (n = 44). Prior to randomization, all patients underwent an initial observation period to confirm the need for RBC transfusion and to identify the hemoglobin concentration (the "set-point") which would define each patient's hemoglobin stabilization and transfusion outcomes. The hemoglobin set-point was less than or equal to 9 g/dL in patients with symptoms and was less than or equal to 7 g/dL in patients without symptoms. Endpoints related to hemolysis included the numbers of patients achieving hemoglobin stabilization, the number of RBC units transfused, [[fatigue]], and health-related quality of life. To achieve a designation of hemoglobin stabilization, a patient had to maintain a hemoglobin concentration above the hemoglobin set-point and avoid any RBC transfusion for the entire 26 week period. Hemolysis was monitored mainly by the measurement of serum LDH levels, and the proportion of PNH RBCs was monitored by flow cytometry. Patients receiving anticoagulants and systemic corticosteroids at baseline continued these medications. | ||
[[File:Eculizumab patient baseline characteristics.png|thumb|none|600px]] | [[File:Eculizumab patient baseline characteristics.png|thumb|none|600px]] | ||
Patients treated with | Patients treated with eculizumab had significantly reduced (p< 0.001) hemolysis resulting in improvements in anemia as indicated by increased hemoglobin stabilization and reduced need for RBC [[transfusions]] compared to placebo treated patients (see Table 9). These effects were seen among patients within each of the three pre-study RBC transfusion strata (4 - 14 units; 15 - 25 units; > 25 units). After 3 weeks of eculizumab treatment, patients reported less fatigue and improved health-related quality of life. Because of the study sample size and duration, the effects of eculizumab on thrombotic events could not be determined. | ||
Percentage of patients with stabilized hemoglobin levels: | Percentage of patients with stabilized hemoglobin levels: | ||
| Line 209: | Line 208: | ||
======Study 2 and Extension Study====== | ======Study 2 and Extension Study====== | ||
PNH patients with at least one transfusion in the prior 24 months and at least 30,000 platelets/microliter received | PNH patients with at least one transfusion in the prior 24 months and at least 30,000 platelets/microliter received eculizumab over a 52-week period. Concomitant medications included [[anti-thrombotic agents]] in 63% of the patients and [[systemic corticosteroids]] in 40% of the patients. Overall, 96 of the 97 enrolled patients completed the study (one patient died following a thrombotic event). A reduction in intravascular [[hemolysis]] as measured by serum LDH levels was sustained for the treatment period and resulted in a reduced need for RBC [[transfusion]] and less [[fatigue]]. 187 eculizumab-treated PNH patients were enrolled in a long term extension study. All patients sustained a reduction in intravascular [[hemolysis]] over a total eculizumab exposure time ranging from 10 to 54 months. There were fewer [[thrombotic events]] with eculizumab treatment than during the same period of time prior to treatment. However, the majority of patients received concomitant anticoagulants; the effects of anticoagulant withdrawal during eculizumab therapy was not studied. | ||
====aHUS==== | ====aHUS==== | ||
Five single-arm studies [four prospective (aHUS Studies 1, 2, 4 and 5) and one retrospective (aHUS Study 3)] evaluated the safety and efficacy of | Five single-arm studies [four prospective (aHUS Studies 1, 2, 4 and 5) and one retrospective (aHUS Study 3)] evaluated the safety and efficacy of eculizumab for the treatment of aHUS. Patients with aHUS received meningococcal vaccination prior to receipt of eculizumab or received prophylactic treatment with antibiotics until 2 weeks after vaccination. In all studies, the dose of eculizumab in adult and adolescent patients was 900 mg every 7 ± 2 days for 4 weeks, followed by 1200 mg 7 ± 2 days later, then 1200 mg every 14 ± 2 days thereafter. The dosage regimen for pediatric patients weighing less than 40 kg enrolled in aHUS Study 3 and Study 5 was based on body weight. Efficacy evaluations were based on thrombotic microangiopathy (TMA) endpoints. | ||
Endpoints related to TMA included the following: | Endpoints related to TMA included the following: | ||
* platelet count change from baseline | * [[platelet count]] change from baseline | ||
* hematologic normalization (maintenance of normal platelet counts and LDH levels for at least four weeks) | * hematologic normalization (maintenance of normal platelet counts and LDH levels for at least four weeks) | ||
* complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatinine for a minimum of four weeks) | * complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatinine for a minimum of four weeks) | ||
| Line 222: | Line 221: | ||
======aHUS Resistant to PE/PI (aHUS Study 1)====== | ======aHUS Resistant to PE/PI (aHUS Study 1)====== | ||
aHUS Study 1 enrolled patients who displayed signs of thrombotic microangiopathy (TMA) despite receiving at least four PE/PI treatments the week prior to screening. One patient had no PE/PI the week prior to screening because of PE/PI intolerance. In order to qualify for enrollment, patients were required to have a platelet count ≤150 x 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 28 (range: 17 to 68 years). Patients enrolled in aHUS Study 1 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 70%-121%. Seventy-six percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 10 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 1. | aHUS Study 1 enrolled patients who displayed signs of [[thrombotic microangiopathy]] (TMA) despite receiving at least four PE/PI treatments the week prior to screening. One patient had no PE/PI the week prior to screening because of PE/PI intolerance. In order to qualify for enrollment, patients were required to have a platelet count ≤150 x 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 28 (range: 17 to 68 years). Patients enrolled in aHUS Study 1 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 70%-121%. Seventy-six percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 10 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 1. | ||
[[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 1.png|thumb|none|600px]] | [[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 1.png|thumb|none|600px]] | ||
Patients in aHUS Study 1 received | Patients in aHUS Study 1 received eculizumab for a minimum of 26 weeks. In aHUS Study 1, the median duration of eculizumab therapy was approximately 100 weeks (range: 2 weeks to 145 weeks). | ||
Renal function, as measured by eGFR, was improved and maintained during | Renal function, as measured by eGFR, was improved and maintained during eculizumab therapy. The mean eGFR (± SD) increased from 23 ± 15 mL/min/1.73m2 at baseline to 56 ± 40 mL/min/1.73m2 by 26 weeks; this effect was maintained through 2 years (56 ± 30 mL/min/1.73m2). Four of the five patients who required dialysis at baseline were able to discontinue dialysis. | ||
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of | Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of eculizumab. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 1, mean platelet count (± SD) increased from 109 ± 32 x109/L at baseline to 169 ± 72 x109/L by one week; this effect was maintained through 26 weeks (210 ± 68 x109/L), and 2 years (205 ± 46 x109/L). When treatment was continued for more than 26 weeks, two additional patients achieved Hematologic Normalization as well as Complete TMA response. Hematologic Normalization and Complete TMA response were maintained by all responders. In aHUS Study 1, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins. | ||
[[File:Eculizumab efficacy results for aHUS study 1.png|thumb|none|600px]] | [[File:Eculizumab efficacy results for aHUS study 1.png|thumb|none|600px]] | ||
======aHUS Sensitive to PE/PI (aHUS Study 2)====== | ======aHUS Sensitive to PE/PI (aHUS Study 2)====== | ||
aHUS Study 2 enrolled patients undergoing chronic PE/PI who generally did not display hematologic signs of ongoing thrombotic microangiopathy (TMA). All patients had received PT at least once every two weeks, but no more than three times per week, for a minimum of eight weeks prior to the first | aHUS Study 2 enrolled patients undergoing chronic PE/PI who generally did not display hematologic signs of ongoing [[thrombotic microangiopathy]] (TMA). All patients had received PT at least once every two weeks, but no more than three times per week, for a minimum of eight weeks prior to the first eculizumab dose. Patients on chronic dialysis were permitted to enroll in aHUS Study 2. The median patient age was 28 years (range: 13 to 63 years). Patients enrolled in aHUS Study 2 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 37%-118%. Seventy percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 12 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 2. | ||
[[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 2.png|thumb|none|600px]] | [[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 2.png|thumb|none|600px]] | ||
Patients in aHUS Study 2 received | Patients in aHUS Study 2 received eculizumab for a minimum of 26 weeks. In aHUS Study 2, the median duration of eculizumab therapy was approximately 114 weeks (range: 26 to 129 weeks). | ||
Renal function, as measured by eGFR, was maintained during | Renal function, as measured by eGFR, was maintained during eculizumab therapy. The mean eGFR (± SD) was 31 ± 19 mL/min/1.73m2 at baseline, and was maintained through 26 weeks (37 ± 21 mL/min/1.73m2) and 2 years (40 ± 18 mL/min/1.73m2). No patient required new dialysis with eculizumab. | ||
Reduction in terminal complement activity was observed in all patients after the commencement of | Reduction in terminal complement activity was observed in all patients after the commencement of eculizumab. Eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. Platelet counts were maintained at normal levels despite the elimination of PE/PI. The mean platelet count (± SD) was 228 ± 78 x 109/L at baseline, 233 ± 69 x 109/L at week 26, and 224 ± 52 x 109/L at 2 years. When treatment was continued for more than 26 weeks, six additional patients achieved Complete TMA response. Complete TMA Response and Hematologic Normalization were maintained by all responders. In aHUS Study 2, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins. | ||
[[File:Eculizumab efficacy results for aHUS study 2.png|thumb|none|600px]] | [[File:Eculizumab efficacy results for aHUS study 2.png|thumb|none|600px]] | ||
======Retrospective Study in Patients with aHUS (aHUS Study 3)====== | ======Retrospective Study in Patients with aHUS (aHUS Study 3)====== | ||
The efficacy results for the aHUS retrospective study (aHUS Study 3) were generally consistent with results of the two prospective studies. | The efficacy results for the aHUS retrospective study (aHUS Study 3) were generally consistent with results of the two prospective studies. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline. Mean platelet count (± SD) increased from 171 ± 83 x109/L at baseline to 233 ±109 x109/L after one week of therapy; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 254 ± 79 x109/L). | ||
A total of 19 pediatric patients (ages 2 months to 17 years) received | A total of 19 pediatric patients (ages 2 months to 17 years) received eculizumab in aHUS Study 3. The median duration of eculizumab therapy was 16 weeks (range 4 to 70 weeks) for children <2 years of age (n=5), 31 weeks (range 19 to 63 weeks) for children 2 to <12 years of age (n=10), and 38 weeks (range 1 to 69 weeks) for patients 12 to <18 years of age (n=4). Fifty three percent of pediatric patients had an identified complement regulatory factor mutation or auto-antibody. | ||
Overall, the efficacy results for these pediatric patients appeared consistent with what was observed in patients enrolled in aHUS Studies 1 and 2 (Table 14). No pediatric patient required new dialysis during treatment with | Overall, the efficacy results for these pediatric patients appeared consistent with what was observed in patients enrolled in aHUS Studies 1 and 2 (Table 14). No pediatric patient required new dialysis during treatment with eculizumab. | ||
[[File:Eculizumab efficacy results in pediatric patients enrolled in a aHUS study 3.png|thumb|none|600px]] | [[File:Eculizumab efficacy results in pediatric patients enrolled in a aHUS study 3.png|thumb|none|600px]] | ||
| Line 261: | Line 260: | ||
[[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 4.png|thumb|none|500px]] | [[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 4.png|thumb|none|500px]] | ||
Patients in aHUS Study 4 received | Patients in aHUS Study 4 received eculizumab for a minimum of 26 weeks. In aHUS Study 4, the median duration of eculizumab therapy was approximately 50 weeks (range: 13 weeks to 86 weeks). | ||
Renal function, as measured by eGFR, was improved during | Renal function, as measured by eGFR, was improved during eculizumab therapy. The mean eGFR (± SD) increased from 17 ± 12 mL/min/1.73m2 at baseline to 47 ± 24 mL/min/1.73m2 by 26 weeks. Twenty of the 24 patients who required dialysis at study baseline were able to discontinue dialysis during eculizumab treatment. | ||
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of | Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of eculizumab. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 4, mean platelet count (± SD) increased from 119 ± 66 x109/L at baseline to 200 ± 84 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 252 ± 70 x109/L). In aHUS Study 4, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H. | ||
[[File:Eculizumab efficacy results for aHUS study 4.png|thumb|none|500px]] | [[File:Eculizumab efficacy results for aHUS study 4.png|thumb|none|500px]] | ||
| Line 274: | Line 273: | ||
[[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 5.png|thumb|none|500px]] | [[File:Eculizumab baseline characteristics of patients enrolled in aHUS study 5.png|thumb|none|500px]] | ||
Patients in aHUS Study 5 received | Patients in aHUS Study 5 received eculizumab for a minimum of 26 weeks. In aHUS Study 5, the median duration of eculizumab therapy was approximately 44 weeks (range: 1 dose to 88 weeks). | ||
Renal function, as measured by eGFR, was improved during | Renal function, as measured by eGFR, was improved during eculizumab therapy. The mean eGFR (± SD) increased from 33 ± 30 mL/min/1.73m2 at baseline to 98 ± 44 mL/min/1.73m2 by 26 weeks. Among the 20 patients with a CKD stage ≥2 at baseline, 17 (85%) achieved a CKD improvement of ≥1 stage. Among the 16 patients ages 1 month to <12 years with a CKD stage ≥2 at baseline, 14 (88%) achieved a CKD improvement by ≥1 stage. Nine of the 11 patients who required dialysis at study baseline were able to discontinue dialysis during eculizumab treatment. Responses were observed across all ages from 5 months to 17 years of age. | ||
Reduction in terminal complement activity was observed in all patients after commencement of | Reduction in terminal complement activity was observed in all patients after commencement of eculizumab. Eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. The mean platelet count (± SD) increased from 88 ± 42 x109/L at baseline to 281 ± 123 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (±SD) at week 26: 293 ± 106 x109/L). In aHUS Study 5, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H. | ||
[[File:Eculizumab efficacy results for aHUS study 5.png|thumb|none|500px]] | [[File:Eculizumab efficacy results for aHUS study 5.png|thumb|none|500px]] | ||
|howSupplied=Eculizumab is supplied as 300 mg single-use vials containing 30 mL of 10 mg/mL sterile, preservative-free | |howSupplied=Eculizumab is supplied as 300 mg single-use vials containing 30 mL of 10 mg/mL sterile, preservative-free eculizumab solution per vial. | ||
|storage=Store at 2-8º C (36-46º F) and protected from light. | |storage=Store at 2-8º C (36-46º F) and protected from light. | ||
|fdaPatientInfo=Prior to treatment, patients should fully understand the risks and benefits of | |packLabel=[[File:Eculizumab FDA package label.png|thumb|none|600px]] | ||
|fdaPatientInfo=Prior to treatment, patients should fully understand the risks and benefits of eculizumab, in particular the risk of meningococcal infection. Ensure that patients receive the Medication Guide. | |||
Inform patients that they are required to receive a meningococcal vaccination at least 2 weeks prior to receiving the first dose of | Inform patients that they are required to receive a meningococcal vaccination at least 2 weeks prior to receiving the first dose of eculizumab, if they have not previously been vaccinated. They are required to be revaccinated according to current medical guidelines for meningococcal vaccine use while on eculizumab therapy. Inform patients that vaccination may not prevent meningococcal infection. Inform patients about the signs and symptoms of meningococcal infection, and strongly advise patients to seek immediate medical attention if these signs or symptoms occur. These signs and symptoms are as follows: | ||
* headache with nausea or vomiting | * [[headache]] with [[nausea]] or [[vomiting]] | ||
* headache and | * [[headache]] and [[fever]] | ||
* headache with a stiff neck or stiff back | * [[headache]] with a [[stiff neck]] or stiff back | ||
* fever of 103° F (39.4° C) or higher | * [[fever]] of 103° F (39.4° C) or higher | ||
* fever and a rash | * [[fever]] and a [[rash]] | ||
* confusion | * [[confusion]] | ||
* muscle aches with flu-like symptoms | * [[muscle aches]] with flu-like symptoms | ||
* eyes sensitive to light | * eyes sensitive to light | ||
Inform patients that they will be given a | Inform patients that they will be given a eculizumab Patient Safety Information Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation. | ||
Inform patients that there may be an increased risk of other types of infections, particularly those due to encapsulated bacteria. Additionally, Aspergillus infections have occured in immunocompromised and neutropenic patients. Inform parents or caregivers of children receiving | Inform patients that there may be an increased risk of other types of infections, particularly those due to encapsulated bacteria. Additionally, [[Aspergillus]] infections have occured in immunocompromised and neutropenic patients. Inform parents or caregivers of children receiving eculizumab for the treatment of aHUS that their child should be vaccinated against Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to current medical guidelines. | ||
Inform patients with PNH that they may develop hemolysis due to PNH when | Inform patients with PNH that they may develop hemolysis due to PNH when eculizumab is discontinued and that they will be monitored by their healthcare professional for at least 8 weeks following eculizumab discontinuation. Inform patients with aHUS that there is a potential for TMA complications due to aHUS when eculizumab is discontinued and that they will be monitored by their healthcare professional for at least 12 weeks following eculizumab discontinuation. Inform patients who discontinue eculizumab to keep the eculizumab Patient Safety Information Card with them for three months after the last eculizumab dose, because the increased risk of meningococcal infection persists for several weeks following discontinuation of eculizumab. | ||
|alcohol=Alcohol-Eculizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Eculizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Soliris <ref>{{cite web|url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebcd67fa-b4d1-4a22-b33d-ee8bf6b9c722|title=FDA LABEL: SOLIRIS- eculizumab injection, solution, concentrate}}</ref> | |||
}} | }} | ||
{{ | {{LabelImage | ||
| | |fileName=Eculizumab 10 mg-ml.png | ||
}} | }} | ||
Latest revision as of 19:49, 19 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete Boxed Warning.
Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early.
|
Overview
Eculizumab is a monoclonal antibody that is FDA approved for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and for patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, diarrhea, nausea, vomiting, anemia, backache, headache, insomnia, nasal congestion, nasopharyngitis, respiratory tract infection and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Paroxysmal Nocturnal Hemoglobinuria (PNH)
Eculizumab is indicated for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis. Dosage:
- 600 mg weekly for the first 4 weeks, followed by
- 900 mg for the fifth dose 1 week later, then
- 900 mg every 2 weeks thereafter.
Atypical Hemolytic Uremic Syndrome (aHUS)
Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. Dosage:
- 900 mg weekly for the first 4 weeks, followed by
- 1200 mg for the fifth dose 1 week later, then
- 1200 mg every 2 weeks thereafter.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eculizumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eculizumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Atypical Hemolytic Uremic Syndrome (aHUS)
- Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
- For patients less than 18 years of age, administer eculizumab based upon body weight, according to the following schedule:
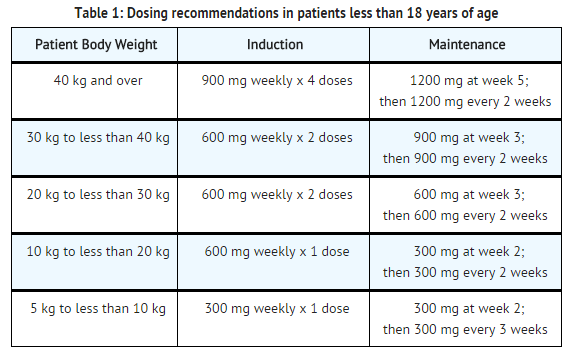
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eculizumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eculizumab in pediatric patients.
Contraindications
- Patients with unresolved serious Neisseria meningitidis infection.
- Patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying eculizumab treatment outweigh the risks of developing a meningococcal infection
Warnings
|
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete Boxed Warning.
Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early.
|
Serious Meningococcal Infections
- The use of eculizumab increases a patient's susceptibility to serious meningococcal infections (septicemia and/or meningitis). Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab.
- Administer a polyvalent meningococcal vaccine according to the most current Advisory Committee on Immunization Practices (ACIP) recommendations for patients with complement deficiencies. Revaccinate patients in accordance with ACIP recommendations, considering the duration of eculizumab therapy.
- Immunize patients without a history of meningococcal vaccination at least 2 weeks prior to receiving the first dose of eculizumab. If urgent eculizumab therapy is indicated in an unvaccinated patient, administer the meningococcal vaccine as soon as possible. In prospective clinical studies, 75/100 patients with aHUS were treated with eculizumab less than 2 weeks after meningococcal vaccination and 64 of these 75 patients received antibiotics for prophylaxis of meningococcal infection until at least 2 weeks after meningococcal vaccination. The benefits and risks of antibiotic prophylaxis for prevention of meningococcal infections in patients receiving eculizumab have not been established.
- Vaccination reduces, but does not eliminate, the risk of meningococcal infections. In clinical studies, 2 out of 196 PNH patients developed serious meningococcal infections while receiving treatment with eculizumab; both had been vaccinated. In clinical studies among non-PNH patients, meningococcal meningitis occurred in one unvaccinated patient. In addition, 3 out of 130 previously vaccinated patients with aHUS developed meningococcal infections while receiving treatment with eculizumab.
- Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if an infection is suspected. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Discontinue eculizumab in patients who are undergoing treatment for serious meningococcal infections.
Eculizumab REMS
- Because of the risk of meningococcal infections, eculizumab is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the eculizumab REMS, prescribers must enroll in the program.
- Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with a meningococcal vaccine.
Other Infections
- eculizumab blocks terminal complement activation; therefore patients may have increased susceptibility to infections, especially with encapsulated bacteria. Additionally, Aspergillus infections have occurred in immunocompromised and neutropenic patients. Children treated with eculizumab may be at increased risk of developing serious infections due to Streptococcuspneumoniae and Haemophilus influenza type b (Hib). Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenza type b (Hib) infections according to ACIP guidelines. Use caution when administering eculizumab to patients with any systemic infection.
Monitoring Disease Manifestations After eculizumab Discontinuation
Treatment Discontinuation for PNH
- Monitor patients after discontinuing eculizumab for at least 8 weeks to detect hemolysis.
Treatment Discontinuation for aHUS
- After discontinuing eculizumab, monitor patients with aHUS for signs and symptoms of thrombotic microangiopathy (TMA) complications for at least 12 weeks. In aHUS clinical trials, 18 patients (5 in the prospective studies) discontinued eculizumab treatment. TMA complications occurred following a missed dose in 5 patients, and eculizumab was reinitiated in 4 of these 5 patients.
Clinical signs and symptoms of TMA include changes in mental status, seizures, angina, dyspnea, or thrombosis. In addition, the following changes in laboratory parameters may identify a TMA complication: occurrence of two, or repeated measurement of any one of the following: a decrease in platelet count by 25% or more compared to baseline or the peak platelet count during eculizumab treatment; an increase in serum creatinine by 25% or more compared to baseline or nadir during eculizumab treatment; or, an increase in serum LDH by 25% or more over baseline or nadir during eculizumab treatment.
If TMA complications occur after eculizumab discontinuation, consider reinstitution of eculizumab treatment, plasma therapy [plasmapheresis, plasma exchange, or fresh frozen plasma infusion (PE/PI)], or appropriate organ-specific supportive measures.
Thrombosis Prevention and Management
- The effect of withdrawal of anticoagulant therapy during eculizumab treatment has not been established. Therefore, treatment with eculizumab should not alter anticoagulant management.
Infusion Reactions
- As with all protein products, administration of eculizumab may result in infusion reactions, including anaphylaxis or other hypersensitivity reactions. In clinical trials, no patients experienced an infusion reaction which required discontinuation of eculizumab. Interrupt eculizumab infusion and institute appropriate supportive measures if signs of cardiovascular instability or respiratory compromise occur.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Meningococcal infections are the most important adverse reactions experienced by patients receiving eculizumab. In PNH clinical studies, two patients experienced meningococcal sepsis. Both patients had previously received a meningococcal vaccine. In clinical studies among patients without PNH, meningococcal meningitis occurred in one unvaccinated patient. Meningococcal sepsis occurred in one previously vaccinated patient enrolled in the retrospective aHUS study during the post-study follow-up period.
PNH
The data described below reflect exposure to eculizumab in 196 adult patients with PNH, age 18-85, of whom 55% were female. All had signs or symptoms of intravascular hemolysis. eculizumab was studied in a placebo-controlled clinical study (in which 43 patients received eculizumab and 44, placebo); a single arm clinical study and a long term extension study. 182 patients were exposed for greater than one year. All patients received the recommended eculizumab dose regimen.
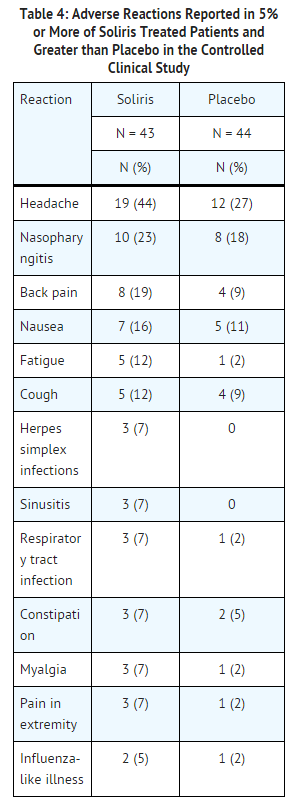
In the placebo-controlled clinical study, serious adverse reactions occurred among 4 (9%) patients receiving eculizumab and 9 (21%) patients receiving placebo. The serious reactions included infections and progression of PNH. No deaths occurred in the study and no patients receiving eculizumab experienced a thrombotic event; one thrombotic event occurred in a patient receiving placebo.
Among 193 patients with PNH treated with eculizumab in the single arm, clinical study or the follow-up study, the adverse reactions were similar to those reported in the placebo-controlled clinical study. Serious adverse reactions occurred among 16% of the patients in these studies. The most common serious adverse reactions were: viral infection (2%), headache (2%), anemia (2%), and pyrexia (2%).
aHUS
The safety of eculizumab therapy in patients with aHUS was evaluated in four prospective, single-arm studies, three in adult and adolescent patients (aHUS Studies 1, 2, and 4), one in pediatric and adolescent patients (aHUS Study 5) and one retrospective study (aHUS Study 3).
The data described below were derived from 78 adult and adolescent patients with aHUS enrolled in aHUS Study 1, aHUS Study 2, and aHUS Study 4. All patients received the recommended dosage of eculizumab. Median exposure was 67 weeks (range: 2-145 weeks). Table 5 summarizes all adverse events reported in at least 10% of patients in aHUS Studies 1, 2, and 4 combined.

In aHUS Studies 1, 2, and 4 combined, 60% (47/78) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were infections (24%), hypertension (5%), chronic renal failure (5%), and renal impairment (5%). Five patients discontinued eculizumab due to adverse events; three due to worsening renal function, one due to new diagnosis of Systemic Lupus Erythematosus, and one due to meningoccal meningitis.
aHUS Study 5 included 22 pediatric and adolescent patients, of which 18 patients were less than 12 years of age. All patients received the recommended dosage of eculizumab. Median exposure was 44 weeks (range: 1 dose-87 weeks).
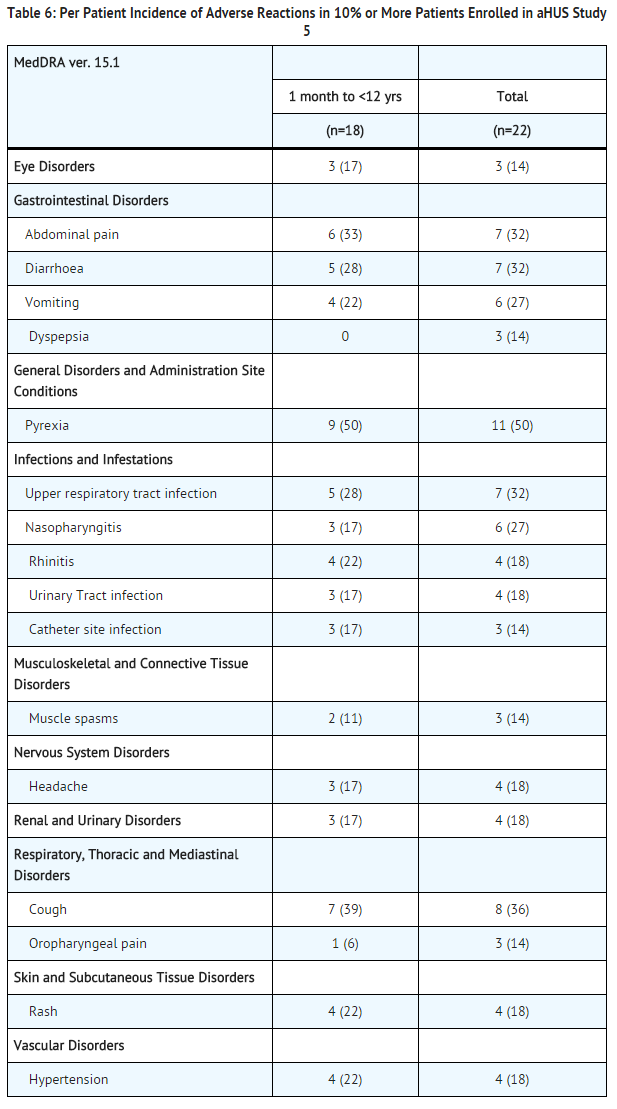
In aHUS Study 5, 59% (13/22) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were hypertension (9%), viral gastroenteritis (9%), pyrexia (9%), and upper respiratory infection (9%). One patient discontinued eculizumab due to an adverse event (severe agitation).
Analysis of retrospectively collected adverse event data from pediatric and adult patients enrolled in aHUS Study 3 (N=30) revealed a safety profile that was similar to that which was observed in the two prospective studies. aHUS Study 3 included 19 pediatric patients less than 18 years of age. Overall, the safety of eculizumab in pediatric patients with aHUS enrolled in Study 3 appeared similar to that observed in adult patients. The most common (≥15%) adverse events occurring in pediatric patients are presented in Table 7.
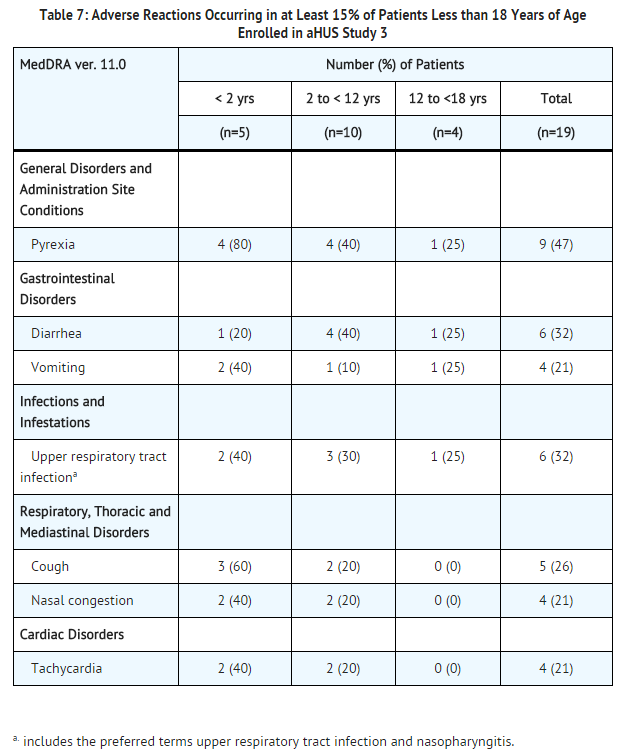
Immunogenicity
As with all proteins, there is a potential for immunogenicity with eculizumab. The immunogenicity of eculizumab has been evaluated using two different immunoassays for the detection of anti-eculizumab antibodies: a direct enzyme-linked immunosorbent assay (ELISA) using the Fab fragment of eculizumab as target was used for the PNH indication; and an electro-chemiluminescence (ECL) bridging assay using the eculizumab whole molecule as target was used for the aHUS indication, as well as for additional patients with PNH. In the PNH population, antibodies to eculizumab were detected in 3/196 (2%) patients with PNH treated with eculizumab using the ELISA assay and in 5/161 (3%) patients treated with eculizumab using the ECL assay. In patients with aHUS treated with eculizumab, antibodies to eculizumab were detected in 3/100 (3%) using the ECL assay. An ECL based neutralizing HAHA assay with a low sensitivity of 2 mcg/mL was performed to detect neutralizing antibodies for the 3 patients with aHUS and also for the 5 patients with PNH with positive samples using the ECL assay. 2/161 patients in the PNH group (1.2%) and 1/100 patients in the aHUS group (1%) had low positive values for neutralizing antibodies. No apparent correlation of antibody development to clinical response was observed in either indication. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to eculizumab in an ELISA-based assay and/or an ECL-based assay and are highly dependent on the sensitivity and specificity of the assay used. Additionally, the observed incidence of antibody positivity in the assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to eculizumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of eculizumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to eculizumab exposure.
Cases of serious or fatal meningococcal infections have been reported.
Drug Interactions
Drug interaction studies have not been performed with eculizumab.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies of eculizumab in pregnant women. eculizumab, a recombinant IgG molecule (humanized anti-C5 antibody), is expected to cross the placenta. Animal studies using a mouse analogue of the eculizumab molecule (murine anti-C5 antibody) showed increased rates of developmental abnormalities and an increased rate of dead and moribund offspring at doses 2-8 times the human dose. eculizumab should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 2-4 times (low dose) and 4-8 times (high dose) the recommended human eculizumab dose, based on a body weight comparison. When animal exposure to the antibody occurred in the time period from before mating until early gestation, no decrease in fertility or reproductive performance was observed. When maternal exposure to the antibody occurred during organogenesis, two cases of retinal dysplasia and one case of umbilical hernia were observed among 230 offspring born to mothers exposed to the higher antibody dose; however, the exposure did not increase fetal loss or neonatal death. When maternal exposure to the antibody occurred in the time period from implantation through weaning, a higher number of male offspring became moribund or died (1/25 controls, 2/25 low dose group, 5/25 high dose group). Surviving offspring had normal development and reproductive function.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eculizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eculizumab during labor and delivery.
Nursing Mothers
It is not known whether eculizumab is excreted into human milk. IgG is excreted in human milk, so it is expected that eculizumab will be present in human milk. However, published data suggest that antibodies in human milk do not enter the neonatal and infant circulation in substantial amounts. Caution should be exercised when eculizumab is administered to a nursing woman. The unknown risks to the infant from gastrointestinal or limited systemic exposure to eculizumab should be weighed against the known benefits of human milk feeding.
Pediatric Use
The safety and effectiveness of eculizumab for the treatment of PNH in pediatric patients below the age of 18 years have not been established.
Four clinical studies assessing the safety and effectiveness of eculizumab for the treatment of aHUS included a total of 47 pediatric patients (ages 2 months to 17 years). The safety and effectiveness of eculizumab for the treatment of aHUS appear similar in pediatric and adult patients.
Administer vaccinations for the prevention of infection due to Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to ACIP guidelines.
Geriatic Use
Nineteen patients 65 years of age or older (15 with PNH and 4 with aHUS) were treated with eculizumab. Although there were no apparent age-related differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Eculizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Eculizumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Eculizumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Eculizumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
Effects of eculizumab upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 4-8 times the equivalent of the clinical dose of eculizumab had no adverse effects on mating or fertility.
Immunocompromised Patients
There is no FDA guidance one the use of Eculizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Eculizumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Eculizumab and IV administrations.
Overdosage
No cases of eculizumab overdose have been reported during clinical studies.
Pharmacology
Eculizumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | Complement protein C5 |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | 148 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 8 to 15 days (mean 11 days) |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
C(US) |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous infusion |
Mechanism of Action
Eculizumab, the active ingredient in eculizumab, is a monoclonal antibody that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9. eculizumab inhibits terminal complement mediated intravascular hemolysis in PNH patients and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
A genetic mutation in patients with PNH leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors, rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia), and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots.
In aHUS, impairment in the regulation of complement activity leads to uncontrolled terminal complement activation, resulting in platelet activation, endothelial cell damage and thrombotic microangiopathy.
Structure
Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa.
Pharmacodynamics
In the PNH placebo-controlled clinical study, eculizumab when administered as recommended reduced hemolysis as shown by the reduction of serum LDH levels from 2200 ± 1034 U/L (mean ± SD) at baseline to 700 ± 388 U/L by week one and maintained the effect through the end of the study at week 26 (327 ± 433 U/L). In the single arm clinical study, eculizumab maintained this effect through 52 weeks.
Pharmacokinetics
A population PK analysis with a standard 1-compartmental model was conducted on the multiple dose PK data from 40 PNH patients receiving the recommended eculizumab regimen. In this model, the clearance of eculizumab for a typical PNH patient weighing 70 kg was 22 mL/hr and the volume of distribution was 7.7 L. The half-life was 272 ± 82 hrs (mean ± SD). The mean observed peak and trough serum concentrations of eculizumab by week 26 were 194 ± 76 mcg/mL and 97 ± 60 mcg/mL, respectively.
A second population PK analysis with a standard 1 compartmental model was conducted on the multiple dose PK data from 57 aHUS patients receiving the recommended eculizumab regimen in studies 1, 2 and 3. In this model, the clearance of eculizumab for a typical aHUS patient weighing 70 kg was 14.6 mL/hr and the volume of distribution was 6.14 L. The elimination half-life was 291 h (approximately 12.1 days).
The clearance and half-life of eculizumab were also evaluated during plasma exchange interventions. Plasma exchange increased the clearance of eculizumab to 3660 mL/hr and reduced the half-life to 1.26 hours. Supplemental dosing is recommended when eculizumab is administered to aHUS patients receiving plasma infusion or exchange.
Dedicated studies have not been conducted to evaluate the PK of eculizumab in special patient populations identified by gender, race, age (geriatric), or the presence of renal or hepatic impairment. Pediatric and adolescent patients (less than 18 years of age) and patients with renal impairment were included in the aHUS clinical studies. Population PK analysis showed age, gender, race, and renal function do not influence the PK of eculizumab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis
- Long-term animal carcinogenicity studies of eculizumab have not been conducted.
- Genotoxicity studies have not been conducted with eculizumab.
Clinical Studies
PNH
The safety and efficacy of eculizumab in PNH patients with hemolysis were assessed in a randomized, double-blind, placebo-controlled 26 week study (Study 1); PNH patients were also treated with eculizumab in a single arm 52 week study (Study 2); and in a long term extension study. Patients received meningococcal vaccination prior to receipt of eculizumab. In all studies, the dose of eculizumab was 600 mg study drug every 7 ± 2 days for 4 weeks, followed by 900 mg 7 ± 2 days later, then 900 mg every 14 ± 2 days for the study duration. eculizumab was administered as an intravenous infusion over 25 - 45 minutes.
Study 1
PNH patients with at least four transfusions in the prior 12 months, flow cytometric confirmation of at least 10% PNH cells and platelet counts of at least 100,000/microliter were randomized to either eculizumab (n = 43) or placebo (n = 44). Prior to randomization, all patients underwent an initial observation period to confirm the need for RBC transfusion and to identify the hemoglobin concentration (the "set-point") which would define each patient's hemoglobin stabilization and transfusion outcomes. The hemoglobin set-point was less than or equal to 9 g/dL in patients with symptoms and was less than or equal to 7 g/dL in patients without symptoms. Endpoints related to hemolysis included the numbers of patients achieving hemoglobin stabilization, the number of RBC units transfused, fatigue, and health-related quality of life. To achieve a designation of hemoglobin stabilization, a patient had to maintain a hemoglobin concentration above the hemoglobin set-point and avoid any RBC transfusion for the entire 26 week period. Hemolysis was monitored mainly by the measurement of serum LDH levels, and the proportion of PNH RBCs was monitored by flow cytometry. Patients receiving anticoagulants and systemic corticosteroids at baseline continued these medications.
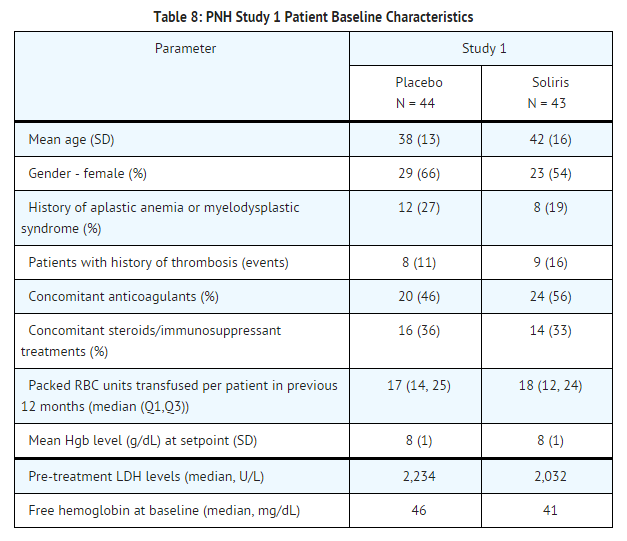
Patients treated with eculizumab had significantly reduced (p< 0.001) hemolysis resulting in improvements in anemia as indicated by increased hemoglobin stabilization and reduced need for RBC transfusions compared to placebo treated patients (see Table 9). These effects were seen among patients within each of the three pre-study RBC transfusion strata (4 - 14 units; 15 - 25 units; > 25 units). After 3 weeks of eculizumab treatment, patients reported less fatigue and improved health-related quality of life. Because of the study sample size and duration, the effects of eculizumab on thrombotic events could not be determined.
Percentage of patients with stabilized hemoglobin levels:
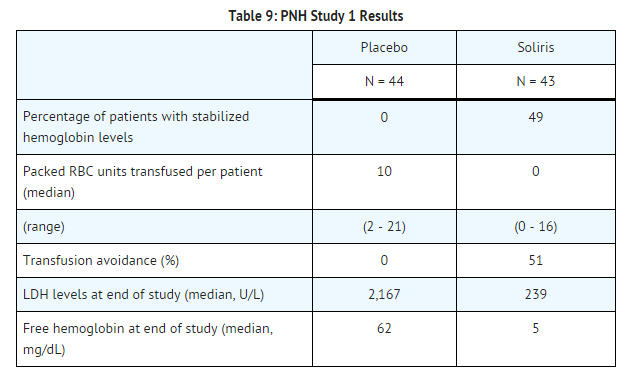
Study 2 and Extension Study
PNH patients with at least one transfusion in the prior 24 months and at least 30,000 platelets/microliter received eculizumab over a 52-week period. Concomitant medications included anti-thrombotic agents in 63% of the patients and systemic corticosteroids in 40% of the patients. Overall, 96 of the 97 enrolled patients completed the study (one patient died following a thrombotic event). A reduction in intravascular hemolysis as measured by serum LDH levels was sustained for the treatment period and resulted in a reduced need for RBC transfusion and less fatigue. 187 eculizumab-treated PNH patients were enrolled in a long term extension study. All patients sustained a reduction in intravascular hemolysis over a total eculizumab exposure time ranging from 10 to 54 months. There were fewer thrombotic events with eculizumab treatment than during the same period of time prior to treatment. However, the majority of patients received concomitant anticoagulants; the effects of anticoagulant withdrawal during eculizumab therapy was not studied.
aHUS
Five single-arm studies [four prospective (aHUS Studies 1, 2, 4 and 5) and one retrospective (aHUS Study 3)] evaluated the safety and efficacy of eculizumab for the treatment of aHUS. Patients with aHUS received meningococcal vaccination prior to receipt of eculizumab or received prophylactic treatment with antibiotics until 2 weeks after vaccination. In all studies, the dose of eculizumab in adult and adolescent patients was 900 mg every 7 ± 2 days for 4 weeks, followed by 1200 mg 7 ± 2 days later, then 1200 mg every 14 ± 2 days thereafter. The dosage regimen for pediatric patients weighing less than 40 kg enrolled in aHUS Study 3 and Study 5 was based on body weight. Efficacy evaluations were based on thrombotic microangiopathy (TMA) endpoints.
Endpoints related to TMA included the following:
- platelet count change from baseline
- hematologic normalization (maintenance of normal platelet counts and LDH levels for at least four weeks)
- complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatinine for a minimum of four weeks)
- TMA-event free status (absence for at least 12 weeks of a decrease in platelet count of >25% from baseline, plasma exchange or plasma infusion, and new dialysis requirement)
- Daily TMA intervention rate (defined as the number of plasma exchange or plasma infusion interventions and the number of new dialyses required per patient per day).
aHUS Resistant to PE/PI (aHUS Study 1)
aHUS Study 1 enrolled patients who displayed signs of thrombotic microangiopathy (TMA) despite receiving at least four PE/PI treatments the week prior to screening. One patient had no PE/PI the week prior to screening because of PE/PI intolerance. In order to qualify for enrollment, patients were required to have a platelet count ≤150 x 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 28 (range: 17 to 68 years). Patients enrolled in aHUS Study 1 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 70%-121%. Seventy-six percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 10 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 1.
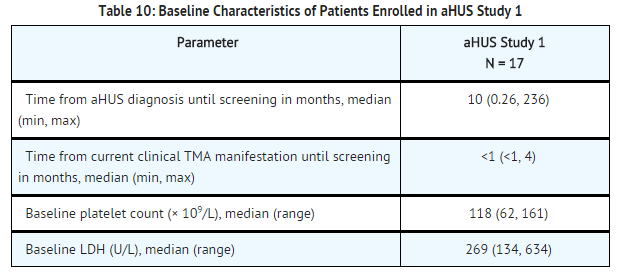
Patients in aHUS Study 1 received eculizumab for a minimum of 26 weeks. In aHUS Study 1, the median duration of eculizumab therapy was approximately 100 weeks (range: 2 weeks to 145 weeks).
Renal function, as measured by eGFR, was improved and maintained during eculizumab therapy. The mean eGFR (± SD) increased from 23 ± 15 mL/min/1.73m2 at baseline to 56 ± 40 mL/min/1.73m2 by 26 weeks; this effect was maintained through 2 years (56 ± 30 mL/min/1.73m2). Four of the five patients who required dialysis at baseline were able to discontinue dialysis.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of eculizumab. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 1, mean platelet count (± SD) increased from 109 ± 32 x109/L at baseline to 169 ± 72 x109/L by one week; this effect was maintained through 26 weeks (210 ± 68 x109/L), and 2 years (205 ± 46 x109/L). When treatment was continued for more than 26 weeks, two additional patients achieved Hematologic Normalization as well as Complete TMA response. Hematologic Normalization and Complete TMA response were maintained by all responders. In aHUS Study 1, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.

aHUS Sensitive to PE/PI (aHUS Study 2)
aHUS Study 2 enrolled patients undergoing chronic PE/PI who generally did not display hematologic signs of ongoing thrombotic microangiopathy (TMA). All patients had received PT at least once every two weeks, but no more than three times per week, for a minimum of eight weeks prior to the first eculizumab dose. Patients on chronic dialysis were permitted to enroll in aHUS Study 2. The median patient age was 28 years (range: 13 to 63 years). Patients enrolled in aHUS Study 2 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 37%-118%. Seventy percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 12 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 2.
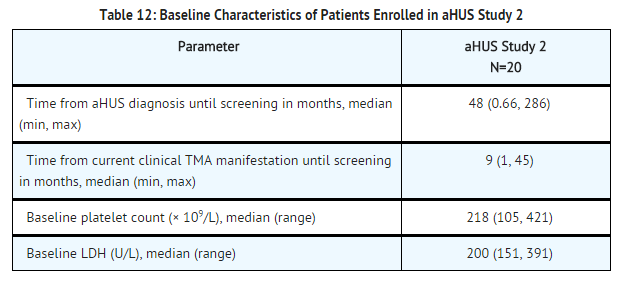
Patients in aHUS Study 2 received eculizumab for a minimum of 26 weeks. In aHUS Study 2, the median duration of eculizumab therapy was approximately 114 weeks (range: 26 to 129 weeks).
Renal function, as measured by eGFR, was maintained during eculizumab therapy. The mean eGFR (± SD) was 31 ± 19 mL/min/1.73m2 at baseline, and was maintained through 26 weeks (37 ± 21 mL/min/1.73m2) and 2 years (40 ± 18 mL/min/1.73m2). No patient required new dialysis with eculizumab.
Reduction in terminal complement activity was observed in all patients after the commencement of eculizumab. Eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. Platelet counts were maintained at normal levels despite the elimination of PE/PI. The mean platelet count (± SD) was 228 ± 78 x 109/L at baseline, 233 ± 69 x 109/L at week 26, and 224 ± 52 x 109/L at 2 years. When treatment was continued for more than 26 weeks, six additional patients achieved Complete TMA response. Complete TMA Response and Hematologic Normalization were maintained by all responders. In aHUS Study 2, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.
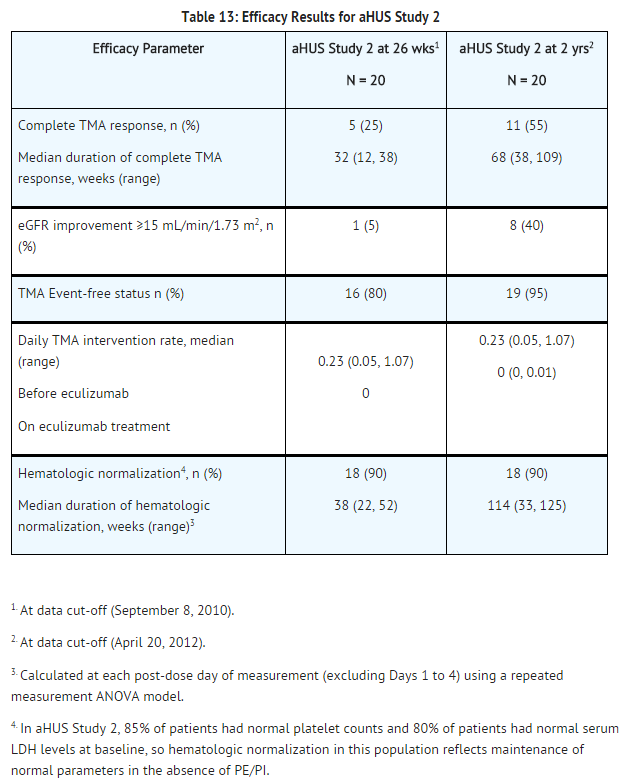
Retrospective Study in Patients with aHUS (aHUS Study 3)
The efficacy results for the aHUS retrospective study (aHUS Study 3) were generally consistent with results of the two prospective studies. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline. Mean platelet count (± SD) increased from 171 ± 83 x109/L at baseline to 233 ±109 x109/L after one week of therapy; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 254 ± 79 x109/L).
A total of 19 pediatric patients (ages 2 months to 17 years) received eculizumab in aHUS Study 3. The median duration of eculizumab therapy was 16 weeks (range 4 to 70 weeks) for children <2 years of age (n=5), 31 weeks (range 19 to 63 weeks) for children 2 to <12 years of age (n=10), and 38 weeks (range 1 to 69 weeks) for patients 12 to <18 years of age (n=4). Fifty three percent of pediatric patients had an identified complement regulatory factor mutation or auto-antibody.
Overall, the efficacy results for these pediatric patients appeared consistent with what was observed in patients enrolled in aHUS Studies 1 and 2 (Table 14). No pediatric patient required new dialysis during treatment with eculizumab.
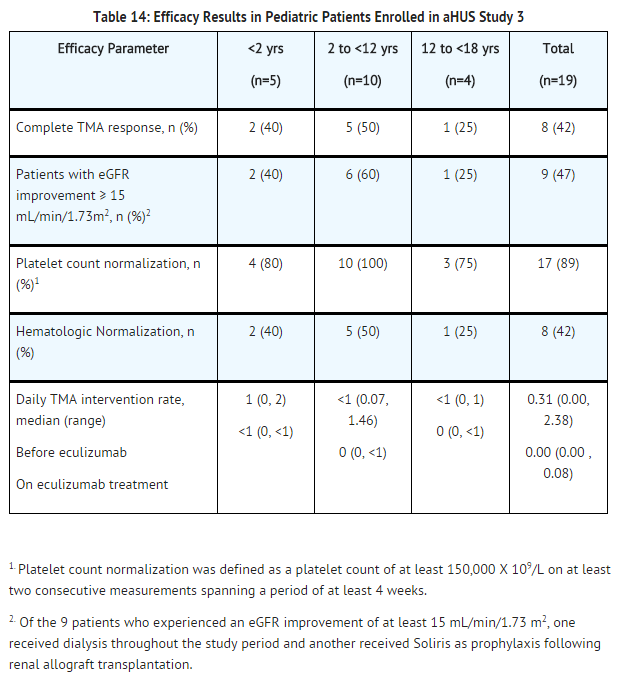
Adult Patients with aHUS (aHUS Study 4)
aHUS Study 4 enrolled patients who displayed signs of thrombotic microangiopathy (TMA). In order to qualify for enrollment, patients were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 35 (range: 18 to 80 years). All patients enrolled in aHUS Study 4 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 28%-116%. Fifty-one percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 35 patients received PE/PI prior to eculizumab. Table 15 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 4.

Patients in aHUS Study 4 received eculizumab for a minimum of 26 weeks. In aHUS Study 4, the median duration of eculizumab therapy was approximately 50 weeks (range: 13 weeks to 86 weeks).
Renal function, as measured by eGFR, was improved during eculizumab therapy. The mean eGFR (± SD) increased from 17 ± 12 mL/min/1.73m2 at baseline to 47 ± 24 mL/min/1.73m2 by 26 weeks. Twenty of the 24 patients who required dialysis at study baseline were able to discontinue dialysis during eculizumab treatment.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of eculizumab. eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 4, mean platelet count (± SD) increased from 119 ± 66 x109/L at baseline to 200 ± 84 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 252 ± 70 x109/L). In aHUS Study 4, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
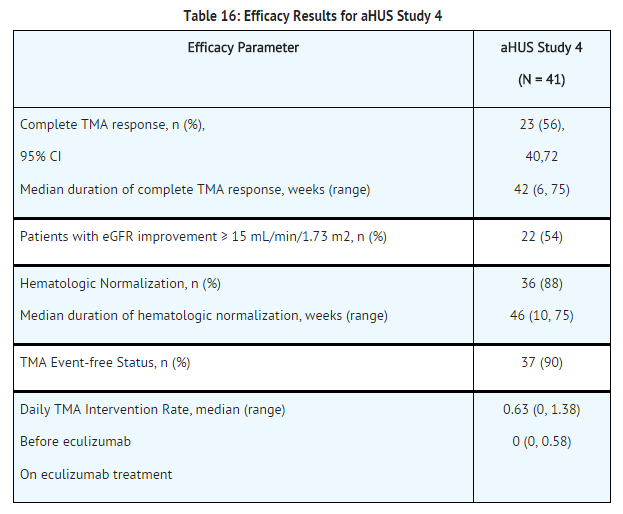
Pediatric and Adolescent Patients with aHUS (aHUS Study 5)
aHUS Study 5 enrolled patients who were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH above the upper limits of normal, serum creatinine level ≥97 percentile for age without the need for chronic dialysis. The median patient age was 6.5 (range: 5 months to 17 years). Patients enrolled in aHUS Study 5 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 38%-121%. Fifty percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 10 patients received PE/PI prior to eculizumab.
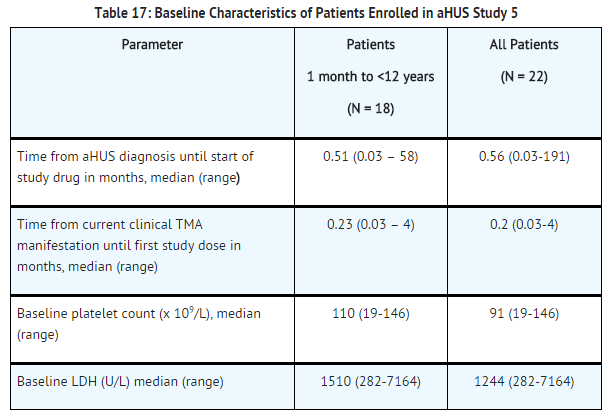
Patients in aHUS Study 5 received eculizumab for a minimum of 26 weeks. In aHUS Study 5, the median duration of eculizumab therapy was approximately 44 weeks (range: 1 dose to 88 weeks).
Renal function, as measured by eGFR, was improved during eculizumab therapy. The mean eGFR (± SD) increased from 33 ± 30 mL/min/1.73m2 at baseline to 98 ± 44 mL/min/1.73m2 by 26 weeks. Among the 20 patients with a CKD stage ≥2 at baseline, 17 (85%) achieved a CKD improvement of ≥1 stage. Among the 16 patients ages 1 month to <12 years with a CKD stage ≥2 at baseline, 14 (88%) achieved a CKD improvement by ≥1 stage. Nine of the 11 patients who required dialysis at study baseline were able to discontinue dialysis during eculizumab treatment. Responses were observed across all ages from 5 months to 17 years of age.
Reduction in terminal complement activity was observed in all patients after commencement of eculizumab. Eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. The mean platelet count (± SD) increased from 88 ± 42 x109/L at baseline to 281 ± 123 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (±SD) at week 26: 293 ± 106 x109/L). In aHUS Study 5, responses to eculizumab were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
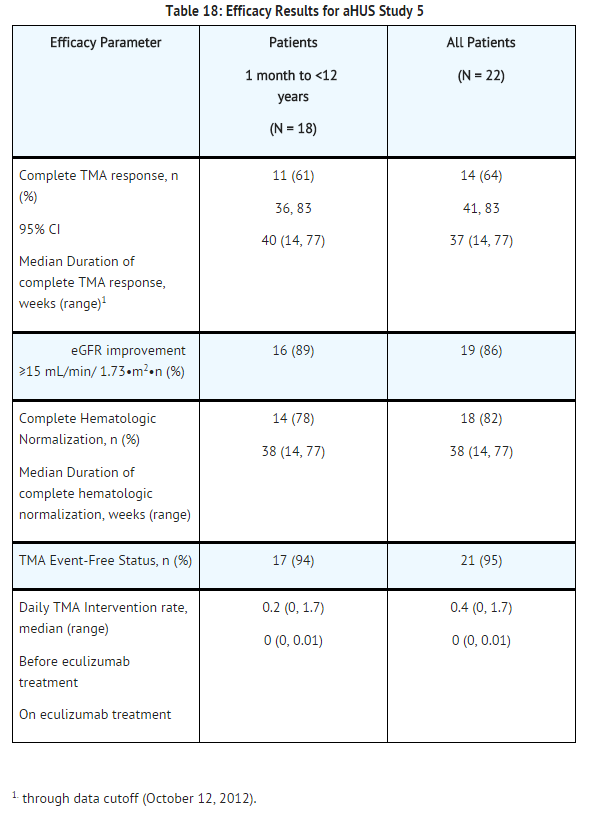
How Supplied
Eculizumab is supplied as 300 mg single-use vials containing 30 mL of 10 mg/mL sterile, preservative-free eculizumab solution per vial.
Storage
Store at 2-8º C (36-46º F) and protected from light.
Images
Drug Images
{{#ask: Page Name::Eculizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eculizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Prior to treatment, patients should fully understand the risks and benefits of eculizumab, in particular the risk of meningococcal infection. Ensure that patients receive the Medication Guide.
Inform patients that they are required to receive a meningococcal vaccination at least 2 weeks prior to receiving the first dose of eculizumab, if they have not previously been vaccinated. They are required to be revaccinated according to current medical guidelines for meningococcal vaccine use while on eculizumab therapy. Inform patients that vaccination may not prevent meningococcal infection. Inform patients about the signs and symptoms of meningococcal infection, and strongly advise patients to seek immediate medical attention if these signs or symptoms occur. These signs and symptoms are as follows:
- headache with nausea or vomiting
- headache and fever
- headache with a stiff neck or stiff back
- fever of 103° F (39.4° C) or higher
- fever and a rash
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Inform patients that they will be given a eculizumab Patient Safety Information Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
Inform patients that there may be an increased risk of other types of infections, particularly those due to encapsulated bacteria. Additionally, Aspergillus infections have occured in immunocompromised and neutropenic patients. Inform parents or caregivers of children receiving eculizumab for the treatment of aHUS that their child should be vaccinated against Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to current medical guidelines.
Inform patients with PNH that they may develop hemolysis due to PNH when eculizumab is discontinued and that they will be monitored by their healthcare professional for at least 8 weeks following eculizumab discontinuation. Inform patients with aHUS that there is a potential for TMA complications due to aHUS when eculizumab is discontinued and that they will be monitored by their healthcare professional for at least 12 weeks following eculizumab discontinuation. Inform patients who discontinue eculizumab to keep the eculizumab Patient Safety Information Card with them for three months after the last eculizumab dose, because the increased risk of meningococcal infection persists for several weeks following discontinuation of eculizumab.
Precautions with Alcohol
Alcohol-Eculizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Soliris [1]
Look-Alike Drug Names
There is limited information regarding Eculizumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Eculizumab |Label Name=Eculizumab 10 mg-ml.png
}}