Dupilumab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 310: | Line 310: | ||
(Description) | (Description) | ||
|howSupplied= | |howSupplied= | ||
|storage=( | *Dupilumab Injection is a clear to slightly opalescent, colorless to pale yellow solution, supplied in single-dose pre-filled syringes with needle shield. Each pre-filled syringe with needle shield is designed to deliver 300 mg of dupilumab in 2 mL solution. | ||
*Dupilumab is available in cartons containing 2 pre-filled syringes with needle shield. | |||
[[image:dupilumabstorage.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|storage= | |||
*Dupilumab is sterile and preservative-free. Discard any unused portion. | |||
*Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light. | |||
*If necessary, pre-filled syringes may be kept at room temperature up to 77°F (25°C) for a maximum of 14 days. Do not store above 77°F (25°C). After removal from the refrigerator, dupilumab must be used within 14 days or discarded. | |||
*Do not expose the syringe to heat or direct sunlight. | |||
*Any unused medicinal product or waste material should be disposed of in accordance with local requirements. | |||
*Do NOT freeze. Do NOT expose to heat. Do NOT shake | |||
|packLabel= | |packLabel= | ||
[[image:dupilumablabel1.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:dupilumablabel1.jpeg|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
Revision as of 16:55, 12 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dupilumab is a interleukin-4 receptor alpha antagonist that is FDA approved for the treatment of moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. Common adverse reactions include injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, and dry eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dupilumab in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Dupilumab and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Dupilumab
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
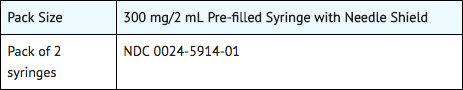
- Dupilumab Injection is a clear to slightly opalescent, colorless to pale yellow solution, supplied in single-dose pre-filled syringes with needle shield. Each pre-filled syringe with needle shield is designed to deliver 300 mg of dupilumab in 2 mL solution.
- Dupilumab is available in cartons containing 2 pre-filled syringes with needle shield.
Storage
- Dupilumab is sterile and preservative-free. Discard any unused portion.
- Store refrigerated at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- If necessary, pre-filled syringes may be kept at room temperature up to 77°F (25°C) for a maximum of 14 days. Do not store above 77°F (25°C). After removal from the refrigerator, dupilumab must be used within 14 days or discarded.
- Do not expose the syringe to heat or direct sunlight.
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
- Do NOT freeze. Do NOT expose to heat. Do NOT shake
Images
Drug Images
{{#ask: Page Name::Dupilumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Dupilumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patients and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use) before the patient starts using dupilumab and each time the prescription is renewed as there may be new information they need to know.
Administration Instructions
- Provide proper training to patients and/or caregivers on proper subcutaneous injection technique, including aseptic technique, and the preparation and administration of dupilumab prior to use. Advise patients to follow sharps disposal recommendations.
Hypersensitivity
- Advise patients to discontinue dupilumab and to seek immediate medical attention if they experience any symptoms of systemic hypersensitivity reactions.
Conjunctivitis and Keratitis
- Advise patients to consult their healthcare provider if new onset or worsening eye symptoms develop.
Comorbid Asthma
- Advise patients with comorbid asthma not to adjust or stop their asthma treatment without talking to their physicians.

Precautions with Alcohol
Alcohol-Dupilumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Dupixent
Look-Alike Drug Names
There is limited information regarding Dupilumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.