Doxazosin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doxazosin is a alpha-adrenergic blocker that is FDA approved for the {{{indicationType}}} of benign prostatic hyperplasia (BPH) and hypertension. Common adverse reactions include edema, hypotension, nausea, dizziness, headache, somnolence, vertigo and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
BENIGN PROSTATIC HYPERPLASIA
- Initial dose:
- 1 mg once a day
- Depending on the patient's urodynamics and BPH symptomatology, dosage may be increased, with a recommended titration interval of 1-2 weeks, to:
- 2 mg once a day, followed by 4 mg once a day until the maximum dosage of 8 mg once a day.
HYPERTENSION
- Initial dose:
- 1 mg once a day
- Depending on the patient's standing blood pressure response (based on measurements taken at 2–6 hours post-dose and 24 hours post-dose), dosage may then be increased to:
- 2 mg once a day, followed by 4 mg once a day, than 8 mg once a day until the maximum dosage of 16 mg once a day to achieve the desired reduction in blood pressure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxazonin sandbox in adult patients.
Non–Guideline-Supported Use
Cardiac syndrome X
Disorder of the urinary system
- Dosing Information
- 2 mg at bedtime, twice daily
Erectile dysfunction; Diagnosis
- Dosing Information
- 4 mg daily
Pheochromocytoma
- Dosing Information
- 2—16 mg daily
Ureteric stone
- Dosing Information
- 4 mg daily
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypertension
- Dosing Information
- Initial: 1 mg/day PO until the maximum dosage of 4 mg/day
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxazosin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxazosin in pediatric patients.
Contraindications
- Doxazosin tablets are contraindicated in patients with a known sensitivity to quinazolines (e.g., prazosin, terazosin), doxazosin, or any of the inert ingredients.
Warnings
Syncope and "First-dose" Effect
- Doxazosin, like other alpha-adrenergic blocking agents, can cause marked hypotension, especially in the upright position, with syncope and other postural symptoms such as dizziness. Marked orthostatic effects are most common with the first dose but can also occur when there is a dosage increase, or if therapy is interrupted for more than a few days. To decrease the likelihood of excessive hypotension and syncope, it is essential that treatment be initiated with the 1 mg dose. The 2 mg, 4 mg, and 8 mg tablets are not for initial therapy. Dosage should then be adjusted slowly with evaluations and increases in dose every two weeks to the recommended dose. Additional antihypertensive agents should be added with caution.
- Patients being titrated with doxazosin should be cautioned to avoid situations where injury could result should syncope occur, during both the day and night.
- If syncope occurs, the patient should be placed in a recumbent position and treated supportively as necessary.
Priapism
- Rarely (probably less frequently than once in every several thousand patients), alpha1 antagonists, including doxazosin, have been associated with priapism (painful penile erection, sustained for hours and unrelieved by sexual intercourse or masturbation). Because this condition can lead to permanent impotence if not promptly treated, patients must be advised about the seriousness of the condition.
Adverse Reactions
Clinical Trials Experience
Benign Prostatic Hyperplasia (BPH)
The incidence of adverse events has been ascertained from worldwide clinical trials in 965 BPH patients. The incidence rates presented below (Table 3) are based on combined data from seven placebo-controlled trials involving once daily administration of doxazosin in doses of 1 mg to 16 mg in hypertensives and 0.5 mg to 8 mg in normotensives. The adverse events when the incidence in the doxazosin group was at least 1% are summarized in Table 3. No significant difference in the incidence of adverse events compared to placebo was seen except for dizziness, fatigue, hypotension, edema and dyspnea. Dizziness and dyspnea appeared to be dose-related.
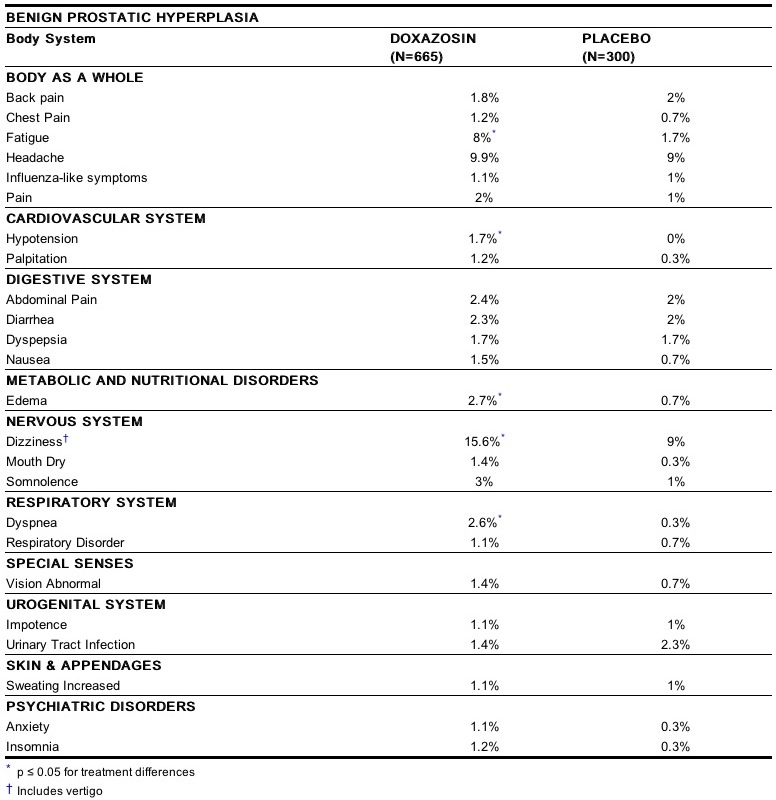
In these placebo-controlled studies of 665 doxazosin patients, treated for a mean of 85 days, additional adverse reactions have been reported. These are less than 1% and not distinguishable from those that occurred in the placebo group. Adverse reactions with an incidence of less than 1% but of clinical interest are (doxazosin vs. placebo): Cardiovascular System: angina pectoris (0.6% vs. 0.7%), postural hypotension (0.3% vs. 0.3%), syncope (0.5% vs. 0%), tachycardia (0.9% vs. 0%); Urogenital System: dysuria (0.5% vs. 1.3%), and Psychiatric Disorders: libido decreased (0.8% vs. 0.3%). The safety profile in patients treated for up to three years was similar to that in the placebo-controlled studies. The majority of adverse experiences with doxazosin were mild.
Hypertension
Doxazosin mesylate has been administered to approximately 4,000 hypertensive patients, of whom 1,679 were included in the hypertension clinical development program. In that program, minor adverse effects were frequent, but led to discontinuation of treatment in only 7% of patients. In placebo-controlled studies adverse effects occurred in 49% and 40% of patients in the doxazosin and placebo groups, respectively, and led to discontinuation in 2% of patients in each group. The major reasons for discontinuation were postural effects (2%), edema, malaise/fatigue, and some heart rate disturbance, each about 0.7%. In controlled hypertension clinical trials directly comparing doxazosin to placebo there was no significant difference in the incidence of side effects, except for dizziness (including postural), weight gain, somnolence and fatigue/malaise. Postural effects and edema appeared to be dose related. The prevalence rates presented below are based on combined data from placebo-controlled studies involving once daily administration of doxazosin at doses ranging from 1 mg to 16 mg. Table 4 summarizes those adverse experiences (possibly/probably related) reported for patients in these hypertension studies where the prevalence rate in the doxazosin group was at least 0.5% or where the reaction is of particular interest.
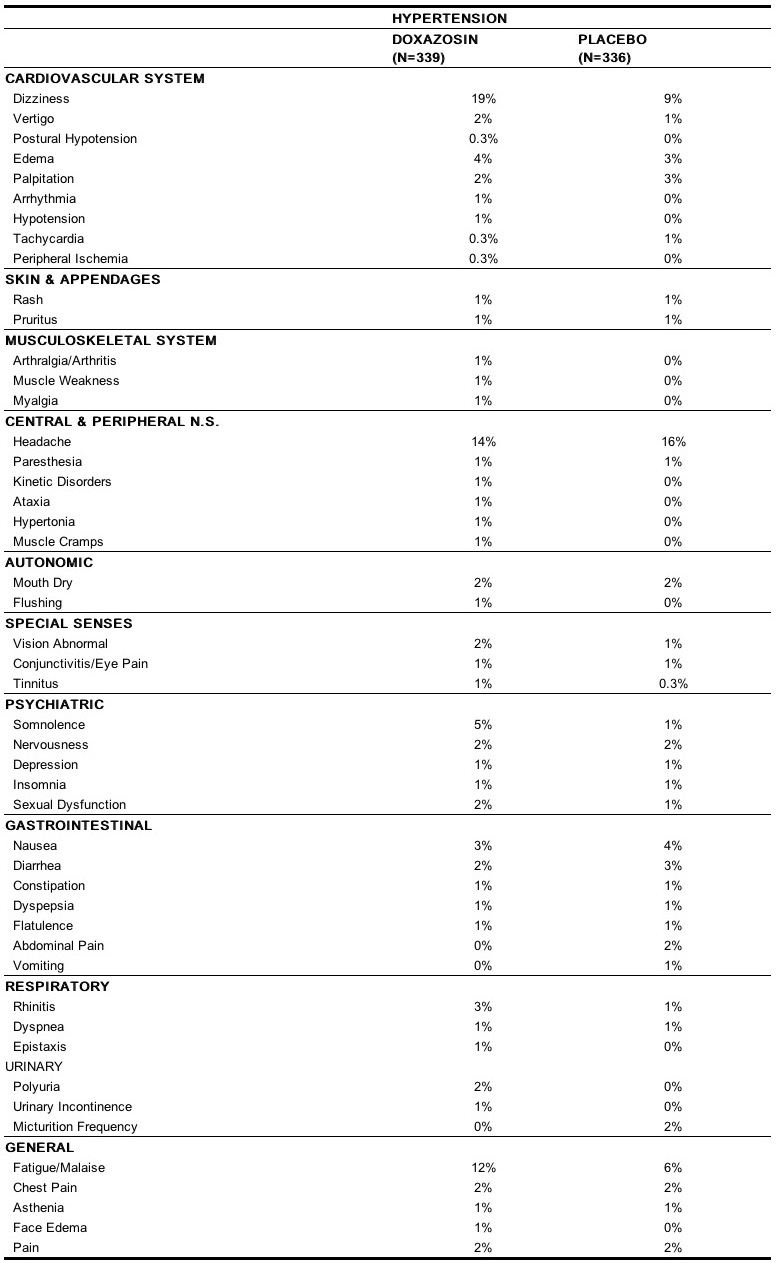
Additional adverse reactions have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to doxazosin. The following adverse reactions occurred with a frequency of between 0.5% and 1%: syncope, hypoesthesia, increased sweating, agitation, increased weight. The following additional adverse reactions were reported by < 0.5% of 3,960 patients who received doxazosin in controlled or open, short- or long-term clinical studies, including international studies. Cardiovascular System: angina pectoris, myocardial infarction, cerebrovascular accident; Autonomic Nervous System: pallor; Metabolic: thirst, gout, hypokalemia; Hematopoietic: lymphadenopathy, purpura; Reproductive System: breast pain; Skin Disorders: alopecia, dry skin, eczema; Central Nervous System: paresis, tremor, twitching, confusion, migraine, impaired concentration; Psychiatric: paroniria, amnesia, emotional lability, abnormal thinking, depersonalization; Special Senses: parosmia, earache, taste perversion, photophobia, abnormal lacrimation; Gastrointestinal System: increased appetite, anorexia, fecal incontinence, gastroenteritis; Respiratory System: bronchospasm, sinusitis, coughing, pharyngitis; Urinary System: renal calculus; General Body System: hot flushes, back pain, infection, fever/rigors, decreased weight, influenza-like symptoms. Doxazosin has not been associated with any clinically significant changes in routine biochemical tests. No clinically relevant adverse effects were noted on serum potassium, serum glucose, uric acid, blood urea nitrogen, creatinine or liver function tests. Doxazosin has been associated with decreases in white blood cell counts.
Postmarketing Experience
- In post-marketing experience the following additional adverse reactions have been reported:
Autonomic Nervous System
Central Nervous System
Endocrine System
Gastrointestinal System
General Body System
Heart Rate/Rhythm
Hematopoietic
Liver/Biliary System
Respiratory System
- Bronchospasm aggravated
Skin Disorders
Special Senses
Urinary System
- Hematuria
- Micturition disorder
- Nocturia
Drug Interactions
Most (98%) of plasma doxazosin is protein bound. In vitro data in human plasma indicate that doxazosin has no effect on protein binding of digoxin, warfarin, phenytoin or indomethacin. There is no information on the effect of other highly plasma protein bound drugs on doxazosin binding. Doxazosin has been administered without any evidence of an adverse drug interaction to patients receiving thiazide diuretics, beta-blocking agents, and nonsteroidal anti-inflammatory drugs. In a placebo-controlled trial in normal volunteers, the administration of a single 1 mg dose of doxazosin on day 1 of a 4-day regimen of oral cimetidine (400 mg twice daily) resulted in a 10% increase in mean AUC of doxazosin (p = 0.006), and a slight but not statistically significant increase in mean Cmax and mean half-life of doxazosin. The clinical significance of this increase in doxazosin AUC is unknown. In clinical trials, doxazosin tablets have been administered to patients on a variety of concomitant medications; while no formal interaction studies have been conducted, no interactions were observed. Doxazosin tablets have been used with the following drugs or drug classes: 1) analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine and codeine combinations, ibuprofen, indomethacin); 2) antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole, amoxicillin); 3) antihistamines (e.g., chlorpheniramine); 4) cardiovascular agents (e.g., atenolol, hydrochlorothiazide, propranolol); 5) corticosteroids; 6) gastrointestinal agents (e.g., antacids); 7) hypoglycemics and endocrine drugs; 8) sedatives and tranquilizers (e.g., diazepam); 9) cold and flu remedies. Concomitant administration of doxazosin tablets with a phosphodiesterase-5 (PDE-5) inhibitor can result in additive blood pressure lowering effects and symptomatic hypotension
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Studies in pregnant rabbits and rats at daily oral doses of up to 41 and 20 mg/kg, respectively (plasma drug concentrations 10 and 4 times human Cmax and AUC exposures with a 12 mg/day therapeutic dose), have revealed no evidence of harm to the fetus. A dosage regimen of 82 mg/kg/day in the rabbit was associated with reduced fetal survival. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, doxazosin should be used during pregnancy only if clearly needed.
Radioactivity was found to cross the placenta following oral administration of labeled doxazosin to pregnant rats.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doxazosin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxazosin during labor and delivery.
Nursing Mothers
Studies in lactating rats given a single oral dose of 1 mg/kg of [2-14C]-doxazosin indicate that doxazosin accumulates in rat breast milk with a maximum concentration about 20 times greater than the maternal plasma concentration. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxazosin is administered to a nursing mother.
Pediatric Use
The safety and effectiveness of doxazosin as an antihypertensive agent have not been established in children.
Geriatic Use
The safety and effectiveness profile of doxazosin in BPH was similar in the elderly (age ≥ 65 years) and younger (age < 65 years) patients.
- For hypertension:
- Clinical studies of doxazosin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Doxazosin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Doxazosin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxazosin in patients with renal impairment.
Hepatic Impairment
Doxazosin should be administered with caution to patients with evidence of impaired hepatic function or to patients receiving drugs known to influence hepatic metabolism
Females of Reproductive Potential and Males
Chronic dietary administration (up to 24 months) of doxazosin mesylate at maximally tolerated doses of 40 mg/kg/day in rats and 120 mg/kg/day in mice revealed no evidence of carcinogenic potential. The highest doses evaluated in the rat and mouse studies are associated with AUCs (a measure of systemic exposure) that are 8 times and 4 times, respectively, the human AUC at a dose of 16 mg/day. Mutagenicity studies revealed no drug or metabolite-related effects at either chromosomal or subchromosomal levels. Studies in rats showed reduced fertility in males treated with doxazosin at oral doses of 20 (but not 5 or 10) mg/kg/day, about 4 times the AUC exposures obtained with a 12 mg/day human dose. This effect was reversible within two weeks of drug withdrawal. There have been no reports of any effects of doxazosin on male fertility in humans.
Immunocompromised Patients
There is no FDA guidance one the use of Doxazosin in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Doxazosin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doxazosin and IV administrations.
Overdosage
- Experience with doxazosin overdosage is limited. Two adolescents who each intentionally ingested 40 mg doxazosin with diclofenac or acetaminophen, were treated with gastric lavage with activated charcoal and made full recoveries. A two-year-old child who accidentally ingested 4 mg doxazosin was treated with gastric lavage and remained normotensive during the five-hour emergency room observation period. A six-month-old child accidentally received a crushed 1 mg tablet of doxazosin and was reported to have been drowsy. A 32-year-old female with chronic renal failure, epilepsy and depression intentionally ingested 60 mg doxazosin (blood level 0.9 mcg/mL; normal values in hypertensives = 0.02 mcg/mL); death was attributed to a grand mal seizure resulting from hypotension. A 39-year-old female who ingested 70 mg doxazosin, alcohol and flurazepam developed hypotension which responded to fluid therapy.
- The oral LD50 of doxazosin is greater than 1000 mg/kg in mice and rats. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of fluid. As doxazosin is highly protein bound, dialysis would not be indicated.
Pharmacology
{| File:Doxazosin.svg | |
| File:Doxazosin ball-and-stick.png | |
| Clinical data | |
|---|---|
| Trade names | Cardura |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693045 |
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 65% |
| Protein binding | 98% |
| Metabolism | Hepatic |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H25N5O5 |
| Molar mass | 451.475 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
There is limited information regarding Doxazosin Mechanism of Action in the drug label.
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Doxazosin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Doxazosin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Doxazosin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Doxazonin sandbox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Doxazosin Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- Pages with script errors
- Pages with broken file links
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Drugboxes which contain changes to watched fields