Doxapram: Difference between revisions
No edit summary |
No edit summary |
||
| Line 26: | Line 26: | ||
Using Single and/or Repeat Single I.V. Injections | Using Single and/or Repeat Single I.V. Injections | ||
Give priming dose of 2 mg/kg body weight and repeat in 5 minutes. The priming dose for moderate depression is 2 mg/kg and the priming dose for mild depression is 1 mg/kg. | *Give priming dose of 2 mg/kg body weight and repeat in 5 minutes. The priming dose for moderate depression is 2 mg/kg and the priming dose for mild depression is 1 mg/kg. | ||
Repeat same dose q 1 to 2h until patient wakens. Watch for relapse into unconsciousness or development of respiratory depression, since DOPRAM does not affect the metabolism of CNS-depressant drugs. | *Repeat same dose q 1 to 2h until patient wakens. Watch for relapse into unconsciousness or development of respiratory depression, since DOPRAM does not affect the metabolism of CNS-depressant drugs. | ||
If relapse occurs, resume injections q 1 to 2h until arousal is sustained, or total maximum daily dose (3 grams) is given. After maximum dose has been given (3 grams), allow patient to sleep until 24 hours have elapsed from first injection of DOPRAM, using assisted or automatic respiration if necessary. | *If relapse occurs, resume injections q 1 to 2h until arousal is sustained, or total maximum daily dose (3 grams) is given. After maximum dose has been given (3 grams), allow patient to sleep until 24 hours have elapsed from first injection of DOPRAM, using assisted or automatic respiration if necessary. | ||
Repeat procedure the following day until patient breathes spontaneously and sustains desired level of consciousness, or until maximum dosage (3 grams) is given. | *Repeat procedure the following day until patient breathes spontaneously and sustains desired level of consciousness, or until maximum dosage (3 grams) is given. | ||
Repetitive doses should be administered only to patients who have shown response to the initial dose. | *Repetitive doses should be administered only to patients who have shown response to the initial dose. | ||
Failure to respond appropriately indicates the need for neurologic evaluation for a possible central nervous system source of sustained coma. | *Failure to respond appropriately indicates the need for neurologic evaluation for a possible central nervous system source of sustained coma. | ||
======Method Two====== | ======Method Two====== | ||
By Intermittent I.V. Infusion | By Intermittent I.V. Infusion. | ||
If patient wakens, watch for relapse; if no response, continue general supportive treatment for 1 to 2 hours and repeat priming dose of DOPRAM. If some respiratory stimulation occurs, prepare I.V. infusion by adding 250 mg of DOPRAM (12.5 mL) to 250 mL of saline or dextrose solution. Deliver at rate of 1 to 3 mg/min (60 to 180 mL/hr) according to size of patient and depth of coma. Discontinue DOPRAM if patient begins to waken or at end of 2 hours. | |||
Continue supportive treatment for ½ to 2 hours and repeat Step b. | *Give priming dose as in Method One. | ||
Do not exceed 3 grams/day. | *If patient wakens, watch for relapse; if no response, continue general supportive treatment for 1 to 2 hours and repeat priming dose of DOPRAM. | ||
*If some respiratory stimulation occurs, prepare I.V. infusion by adding 250 mg of DOPRAM (12.5 mL) to 250 mL of saline or dextrose solution. Deliver at rate of 1 to 3 mg/min (60 to 180 mL/hr) according to size of patient and depth of coma. *Discontinue DOPRAM if patient begins to waken or at end of 2 hours. | |||
*Continue supportive treatment for ½ to 2 hours and repeat Step b. Do not exceed 3 grams/day. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Doxapram in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Doxapram in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Doxapram in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Doxapram in adult patients. | ||
Revision as of 16:15, 3 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doxapram is a respiratory stimulant that is FDA approved for the treatment of Postanesthesia, Drug-Induced Central Nervous System Depression and Chronic Pulmonary Disease Associated with Acute Hypercapnia. Common adverse reactions include flushing, pruritus, diarrhea, nausea, vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Post-Anesthetic Use

By I.V. Injection
The recommended dose for I.V. administration is 0.5 – 1 mg/kg for a single injection and at 5-minute intervals. Careful observation of the patient during administration and for some time subsequently are advisable. The maximum total dosage by I.V. injection is 2 mg/kg.
By Infusion
The solution is prepared by adding 250 mg of doxapram (12.5 mL) to 250 mL of dextrose 5% or 10% in water or normal saline solution. The infusion is initiated at a rate of approximately 5 mg/minute until a satisfactory respiratory response is observed, and maintained at a rate of 1 to 3 mg/minute. The rate of infusion should be adjusted to sustain the desired level of respiratory stimulation with a minimum of side effects. The maximum total dosage by infusion is 4 mg/kg, or approximately 300 mg for the average adult.
Management of Drug-Induced CNS Depression
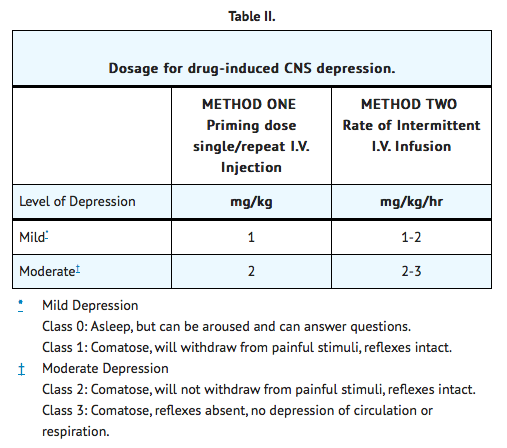
Method One
Using Single and/or Repeat Single I.V. Injections
- Give priming dose of 2 mg/kg body weight and repeat in 5 minutes. The priming dose for moderate depression is 2 mg/kg and the priming dose for mild depression is 1 mg/kg.
- Repeat same dose q 1 to 2h until patient wakens. Watch for relapse into unconsciousness or development of respiratory depression, since DOPRAM does not affect the metabolism of CNS-depressant drugs.
- If relapse occurs, resume injections q 1 to 2h until arousal is sustained, or total maximum daily dose (3 grams) is given. After maximum dose has been given (3 grams), allow patient to sleep until 24 hours have elapsed from first injection of DOPRAM, using assisted or automatic respiration if necessary.
- Repeat procedure the following day until patient breathes spontaneously and sustains desired level of consciousness, or until maximum dosage (3 grams) is given.
- Repetitive doses should be administered only to patients who have shown response to the initial dose.
- Failure to respond appropriately indicates the need for neurologic evaluation for a possible central nervous system source of sustained coma.
Method Two
By Intermittent I.V. Infusion.
- Give priming dose as in Method One.
- If patient wakens, watch for relapse; if no response, continue general supportive treatment for 1 to 2 hours and repeat priming dose of DOPRAM.
- If some respiratory stimulation occurs, prepare I.V. infusion by adding 250 mg of DOPRAM (12.5 mL) to 250 mL of saline or dextrose solution. Deliver at rate of 1 to 3 mg/min (60 to 180 mL/hr) according to size of patient and depth of coma. *Discontinue DOPRAM if patient begins to waken or at end of 2 hours.
- Continue supportive treatment for ½ to 2 hours and repeat Step b. Do not exceed 3 grams/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxapram in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxapram in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Doxapram FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxapram in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxapram in pediatric patients.
Contraindications
- Doxapram is contraindicated in patients with known hypersensitivity to the drug or any of the injection components.
- Doxapram should not be used in patients with epilepsy or other convulsive disorders.
- Doxapram is contraindicated in patients with proven or suspected pulmonary embolism.
- Doxapram is contraindicated in patients with mechanical disorders of ventilation such as mechanical obstruction, muscle paresis (including neuromuscular blockade), flail chest, pneumothorax, acute bronchial asthma, pulmonary fibrosis, or other conditions resulting in restriction of the chest wall, muscles of respiration, or alveolar expansion.
- Doxapram is contraindicated in patients with evidence of head injury, cerebral vascular accident, or cerebral edema, and in those with significant cardiovascular impairment, uncompensated heart failure, severe coronary artery disease, or severe hypertension, including that associated with hyperthyroidism or pheochromocytoma.
Warnings
Doxapram should not be used in conjunction with mechanical ventilation. Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources.
In Postanesthetic Use
Doxapram is neither an antagonist to muscle relaxant drugs nor a specific narcotic antagonist. More specific tests (eg, peripheral nerve stimulation, airway pressures, head lift, pulse oximetry, and end-tidal carbon dioxide) to assess adequacy of ventilation are recommended before administering doxapram. Doxapram should be administered with great care and only under careful supervision to patients with hypermetabolic states such as hyperthyroidism or pheochromocytoma. Since narcosis may recur after stimulation with doxapram, care should be taken to maintain close observation until the patient has been fully alert for ½ to 1 hour. In patients who have received general anesthesia utilizing a volatile agent known to sensitize the myocardium to catecholamines, administration of doxapram should be delayed until the volatile agent has been excreted in order to lessen the potential for arrhythmias, including ventricular tachycardia and ventricular fibrillation.
In Drug-Induced CNS and Respiratory Depression
Doxapram alone may not stimulate adequate spontaneous breathing or provide sufficient arousal in patients who are severely depressed either due to respiratory failure or to CNS depressant drugs, but may be used as an adjunct to established supportive measures and resuscitative techniques.
In Chronic Obstructive Pulmonary Disease
Because of the associated increased work of breathing, do not increase the rate of infusion of doxapram in severely ill patients in an attempt to lower pCO2.
Adverse Reactions
Clinical Trials Experience
Adverse reactions reported coincident with the administration of DOPRAM (doxapram hydrochloride, USP) include:
Central and Autonomic Nervous Systems
Pyrexia, flushing, sweating; pruritus and paresthesia, such as a feeling of warmth, burning, or hot sensation, especially in the area of genitalia and perineum; apprehension, disorientation, pupillary dilatation, hallucinations, headache, dizziness, hyperactivity, involuntary movements, muscle spasticity, muscle fasciculations, increased deep tendon reflexes, clonus, bilateral Babinski, and convulsions.
Respiratory
Dyspnea, cough, hyperventilation, tachypnea, laryngospasm, bronchospasm, hiccough, and rebound hypoventilation.
Cardiovascular
Phlebitis, variations in heart rate, lowered T-waves, arrhythmias (including ventricular tachycardia and ventricular fibrillation), chest pain, tightness in chest. A mild to moderate increase in blood pressure is commonly noted and may be of concern in patients with severe cardiovascular diseases.
Gastrointestinal
Nausea, vomiting, diarrhea, desire to defecate.
Genitourinary
Stimulation of urinary bladder with spontaneous voiding; urinary retention. Elevation of BUN and albuminuria.
Hemic and Lymphatic
Hemolysis with rapid infusion. A decrease in hemoglobin, hematocrit, or red blood cell count has been observed in postoperative patients. In the presence of pre-existing leukopenia, a further decrease in WBC has been observed following anesthesia and treatment with doxapram hydrochloride.
Postmarketing Experience
There is limited information regarding Doxapram Postmarketing Experience in the drug label.
Drug Interactions
- Administration of doxapram to patients who are receiving sympathomimetic or monoamine oxidase inhibiting drugs may result in an additive pressor effect.
- In patients who have received neuromuscular blocking agents, doxapram may temporarily mask the residual effects of these drugs.
- In patients who have received general anesthesia utilizing a volatile agent known to sensitize the myocardium to catecholamines, administration of doxapram should be delayed until the volatile agent has been excreted in order to lessen the potential for arrhythmias, including ventricular tachycardia and ventricular fibrillation.
- There may be an interaction between doxapram and aminophylline and between doxapram and theophylline manifested by increased skeletal muscle activity, agitation, and hyperactivity.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in rats at doses up to 1.6 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to doxapram. There are, however, no adequate and well-controlled studies in pregnant women. Because the animals in the reproduction studies were dosed by the IM and oral routes and animal reproduction studies, in general, are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doxapram in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxapram during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doxapram hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 12 years have not been established. This product contains benzyl alcohol as a preservative. Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The “gasping syndrome”, (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth-weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the “gasping syndrome”, the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
Premature neonates given doxapram have developed hypertension, irritability, jitteriness, hyperglycemia, glucosuria, abdominal distension, increased gastric residuals, vomiting, bloody stools, necrotizing enterocolitis, erratic limb movements, excessive crying, disturbed sleep, premature eruption of teeth, and QT prolongation that has resulted in heart block. In premature neonates with risk factors such as a previous seizure, perinatal asphyxia, or intracerebral hemorrhage, seizures have occurred. In many instances, doxapram was administered following administration of xanthine derivatives such as caffeine, aminophylline or theophylline.
Geriatic Use
There is no FDA guidance on the use of Doxapram in geriatric settings.
Gender
There is no FDA guidance on the use of Doxapram with respect to specific gender populations.
Race
There is no FDA guidance on the use of Doxapram with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxapram in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Doxapram in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Doxapram in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Doxapram in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Doxapram Administration in the drug label.
Monitoring
There is limited information regarding Doxapram Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doxapram and IV administrations.
Overdosage
Signs and Symptoms
Symptoms of overdosage are extensions of the pharmacologic effects of the drug. Excessive pressor effect, such as hypertension, tachycardia, skeletal muscle hyperactivity, and enhanced deep tendon reflexes may be early signs of overdosage. Therefore, the blood pressure, pulse rate, and deep tendon reflexes should be evaluated periodically and the dosage or infusion rate adjusted accordingly.
Other effects may include agitation, confusion, sweating, cough, and dyspnea.
Convulsive seizures are unlikely at recommended dosages. In unanesthetized animals, the convulsant dose is 70 times greater than the respiratory stimulant dose. Intravenous LD50 values in the mouse and rat were approximately 75 mg/kg and in the cat and dog were 40 to 80 mg/kg.
Except for management of chronic obstructive pulmonary disease associated with acute hypercapnia, the maximum recommended dosage is 3 GRAMS/24 HOURS. (See DOSAGE AND ADMINISTRATION.)
Management
There is no specific antidote for doxapram. Management should be symptomatic. Anticonvulsants, along with oxygen and resuscitative equipment should be readily available to manage overdosage manifested by excessive central nervous system stimulation. Slow administration of the drug and careful observation of the patient during administration and for some time subsequently are advisable. These precautions are to assure that the protective reflexes have been restored and to prevent possible post-hyperventilation or hypoventilation.
There is no evidence that doxapram is dialyzable; further, the half-life of doxapram makes it unlikely that dialysis would be appropriate in managing overdose with this drug.
Pharmacology
| |
Doxapram
| |
| Systematic (IUPAC) name | |
| 1-ethyl-4- (2-morpholin-4-ylethyl)- 3,3-diphenyl-pyrrolidin-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | R07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 378.507 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Rx only |
| Routes | Intravenous |
Mechanism of Action
There is limited information regarding Doxapram Mechanism of Action in the drug label.
Structure
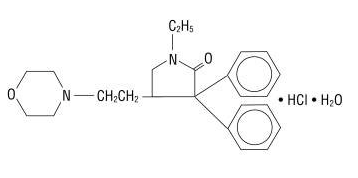
Doxapram hydrochloride is a white to off-white, crystalline powder, sparingly soluble in water, alcohol and chloroform. Chemically, doxapram hydrochloride is 1-ethyl-4-2-(4-morpholinyl)ethyl-3,3-diphenyl-2-pyrrolidinone monohydrochloride, monohydrate.
The chemical structure is:

C24H31ClN2O2 • H2O M.W. 432.98
Pharmacodynamics
Doxapram hydrochloride produces respiratory stimulation mediated through the peripheral carotid chemoreceptors. As the dosage level is increased, the central respiratory centers in the medulla are stimulated with progressive stimulation of other parts of the brain and spinal cord. The onset of respiratory stimulation following the recommended single intravenous injection of doxapram hydrochloride usually occurs in 20 to 40 seconds with peak effect at 1 to 2 minutes. The duration of effect may vary from 5 to 12 minutes. The respiratory stimulant action is manifested by an increase in tidal volume associated with a slight increase in respiratory rate.
A pressor response may result following doxapram administration. Provided there is no impairment of cardiac function, the pressor effect is more marked in hypovolemic state than in normovolemic state. The pressor response is due to the improved cardiac output rather than peripheral vasoconstriction. Following doxapram administration, an increased release of catecholamines has been noted.
Although opiate-induced respiratory depression is antagonized by doxapram, the analgesic effect is not affected.
Pharmacokinetics
Doxapram is metabolized via ring hydroxylation to ketodoxapram, an active metabolite readily detected in the plasma.
Nonclinical Toxicology
No carcinogenic or mutagenic studies have been performed using doxapram. Doxapram did not adversely affect the breeding performance of rats.
Clinical Studies
There is limited information regarding Doxapram Clinical Studies in the drug label.
How Supplied
DOPRAM Injection (doxapram hydrochloride injection, USP) is available in boxes of six 20 mL multiple dose vials containing 20 mg of doxapram hydrochloride per mL with benzyl alcohol 0.9% as the preservative (NDC 60977-144-02).
Storage
Store at Controlled Room Temperature, Between 20˚C to 25˚C (68˚F to 77˚F).
Images
Drug Images
{{#ask: Page Name::Doxapram |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Doxapram |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Doxapram Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Doxapram interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Doxapram Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Doxapram Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.