Dolasetron (injection): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=[[antiemetic]] | |drugClass=[[antiemetic]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=postoperative [[nausea]] and/or [[vomiting]] and for prevention of postoperative [[nausea]] and [[vomiting]] | |indication=postoperative [[nausea]] and/or [[vomiting]] and for prevention of postoperative [[nausea]] and [[vomiting]] | ||
|adverseReactions=[[diarrhea]], [[dizziness]], [[headache]], [[fatigue]], [[urinary retention]], [[pain]], and [[drowsiness]] | |adverseReactions=[[diarrhea]], [[dizziness]], [[headache]], [[fatigue]], [[urinary retention]], [[pain]], and [[drowsiness]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
| Line 92: | Line 92: | ||
* Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs). | * Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs). | ||
|useInPregnancyFDA= | |useInPregnancyFDA='''Teratogenic Effects''' | ||
|useInPregnancyAUS= | '''Pregnancy Category B''' | ||
* Teratology studies have not revealed evidence of impaired fertility or harm to the fetus due to dolasetron mesylate. These studies have been performed in pregnant rats at intravenous doses up to 60 mg/kg/day (5.4 times the recommended human dose based on body surface area) and pregnant rabbits at intravenous doses up to 20 mg/kg/day (3.2 times the recommended human dose based on body surface area). There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether dolasetron mesylate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ANZEMET Injection is administered to a nursing woman. | |||
|useInPed='''Prevention of chemotherapy-induced nausea and vomiting (CINV)''' | |||
* Dolasetron is contraindicated in pediatric patients for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy. | |||
'''Prevention and treatment of post-operative nausea and vomiting (PONV)''' | |||
* Safety and effectiveness in pediatric patients (2 years and older) for prevention and treatment of postoperative nausea and vomiting is based on pharmacokinetic studies and efficacy data in adults. Safety and effectiveness in pediatric patients under 2 years of age have not been established. | |||
Two open-label, noncomparative pharmacokinetic studies have been performed in a total of 30 pediatric patients undergoing surgery with general anesthesia. These patients received ANZEMET Injection either intravenously or orally in juice. Pediatric patients from 2 to 12 years of age participated in these trials, which included an intravenous ANZEMET Injection dose of 1.2 mg/kg, and an oral dose of 1.2 mg/kg. There is no experience in pediatric patients under 2 years of age. Overall, ANZEMET Injection was well tolerated in these pediatric patients. No efficacy information was collected in the pediatric postoperative nausea and vomiting studies. | |||
|useInGeri='''Prevention of chemotherapy-induced nausea and vomiting (CINV)''' | |||
* Dolasetron is contraindicated in geriatric patients for prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy. | |||
'''Prevention and treatment of post-operative nausea and vomiting (PONV)''' | |||
* Controlled clinical studies in the prevention and treatment of post-operative nausea and vomiting did not include sufficient numbers of patients aged 65 years or older – only 57 (2%) geriatric patients (43 received intravenous ANZEMET Injection) out of 3289 total patients participated in the controlled PONV trials – to determine whether they respond differently from younger patients. Other reported clinical experiences have not identified differences in responses between geriatric and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
* Elderly patients are at particular risk for prolongation of the PR, QRS, and QT interval; therefore, caution should be exercised and ECG monitoring should be performed when using ANZEMET for prevention of postoperative nausea and vomiting in this population. | |||
* The pharmacokinetics, including clearance of intravenous ANZEMET Injection, in elderly and younger patients are similar. Dosage adjustment is not needed in patients over the age of 65. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
Revision as of 14:22, 19 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dolasetron (injection) is an antiemetic that is FDA approved for the treatment of postoperative nausea and/or vomiting and for prevention of postoperative nausea and vomiting. Common adverse reactions include diarrhea, dizziness, headache, fatigue, urinary retention, pain, and drowsiness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
ANZEMET Injection is indicated for the following:
The prevention of postoperative nausea and vomiting (PONV) in adults and children 2 years and older
- As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ANZEMET Injection is recommended even where the incidence of postoperative nausea and/or vomiting is low.
- When prophylaxis has failed, a repeat dose should not be initiated as rescue therapy.
The treatment of postoperative nausea and/or vomiting in adults and children 2 years and older
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dolasetron (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dolasetron (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dolasetron (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dolasetron (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dolasetron (injection) in pediatric patients.
Contraindications
- ANZEMET Injection is contraindicated in patients known to have hypersensitivity to the drug.
- ANZEMET Injection solution administered intravenously is contraindicated in adult and pediatric patients for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy due to dose dependent QT prolongation. Mean QTc effects over 20 ms are expected in this patient population
Warnings
QTc Interval Prolongation
- ANZEMET prolongs the QT interval in a dose dependent fashion. Torsade de Pointes has been reported during post-marketing experience. Avoid ANZEMET in patients with congenital long QT syndrome, hypokalemia or hypomagnesemia. Hypokalemia and hypomagnesemia must be corrected prior to ANZEMET administration. Monitor these electrolytes after administration as clinically indicated. Use ECG monitoring in patients with congestive heart failure and bradycardia.
PR and QRS Interval Prolongation
- ANZEMET has been shown to cause dose dependent prolongation of the PR and QRS interval and reports of second or third degree atrioventricular block, cardiac arrest and serious ventricular arrhythmias including fatalities in both adult and pediatric patients. At particular risk are patients with underlying structural heart disease and preexisting conduction system abnormalities, elderly, patients with sick sinus syndrome, patients with atrial fibrillation with slow ventricular response, patients with myocardial ischemia or patients receiving drugs known to prolong the PR interval (such as verapamil) and QRS interval (e.g., flecainide or quinidine). ANZEMET should be used with caution and with ECG monitoring in these patients. ANZEMET should be avoided in patients with complete heart block or at risk for complete heart block, unless they have an implanted pacemaker.
Serotonin Syndrome
- The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post-anesthesia care unit or an infusion center.
- Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of Anzemet and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue Anzemet and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if Anzemet is used concomitantly with other serotonergic drugs
Adverse Reactions
Clinical Trials Experience
Postoperative Patients
- In controlled clinical trials with 2550 adult patients, headache and dizziness were reported more frequently with 12.5 mg ANZEMET Injection than with placebo. Rates of other adverse events were similar. Following is a listing of all adverse events reported in ≥2% of patients receiving either placebo or 12.5 mg ANZEMET Injection for the prevention or treatment of postoperative nausea and vomiting in controlled clinical trials (Table 2).
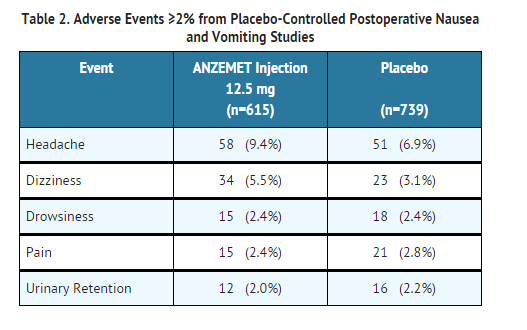
- In clinical trials, the following reported adverse events, assessed by investigators as treatment-related or causality unknown, occurred following oral or intravenous administration of ANZEMET in < 2% of adult patients undergoing surgery:
Body as a Whole: Chills/shivering.
Cardiovascular: Sinus arrhythmia, hypotension, orthostatic hypotension. The following events also occurred and with a similar frequency as placebo and/or active comparator: Mobitz I AV block, chest pain, syncope, severe bradycardia, and palpitations.
In addition, the following asymptomatic treatment-emergent ECG changes were seen at rates less than or equal to those for active or placebo controls: bradycardia, tachycardia, T wave change, ST-T wave change, extrasystole (APCs or VPCs), bundle branch block (left and right).
Furthermore, severe hypotension, bradycardia and syncope have been reported immediately or closely following IV administration.
Dermatologic:Rash
Gastrointestinal System: Constipation, dyspepsia, abdominal pain.
Hearing, Taste and Vision: Taste perversion, abnormal vision.
Hypersensitivity:Anaphylactic reaction, urticaria.
Liver and Biliary System: Transient increases in AST (SGOT) and/or ALT (SGPT). The increases did not appear to be related to dose or duration of therapy and were not associated with symptomatic hepatic disease. Similar increases were seen with patients receiving active comparator.
Musculoskeletal: Myalgia, arthralgia.
Nervous System: Vertigo; flushing, paraesthesia.
Psychiatric: Agitation, anxiety, abnormal dreaming.
Respiratory System: Bronchospasm.
Vascular (Extracardiac): Local pain or burning on IV administration.
Postmarketing Experience
There are reports of wide complex tachycardia or ventricular tachycardia and of ventricular fibrillation cardiac arrest following intravenous administration.
Drug Interactions
- The potential for clinically significant drug-drug interactions posed by dolasetron and hydrodolasetron appears to be low for drugs commonly used in surgery, because hydrodolasetron is eliminated by multiple routes.
- When oral dolasetron (200 mg once daily) was coadministered with cimetidine (300 mg four times daily) for 7 days, the systemic exposure (i.e., AUC) of hydrodolasetron increased by 24% and the maximum plasma concentration of hydrodolasetron increased by 15%. When oral dolasetron (200 mg once daily) was coadministered with rifampin (600 mg once daily) for 7 days, the systemic exposure of hydrodolasetron decreased by 28% and the maximum plasma concentration of hydrodolasetron decreased by 17%.
- Caution should be exercised when ANZEMET Injection is coadministered with drugs that prolong ECG intervals and/or cause hypokalemia or hypomagnesemia.
- In patients taking furosemide, nifedipine, diltiazem, ACE inhibitors, verapamil, glyburide, and propranolol, no effect was shown on the clearance of hydrodolasetron. Clearance of hydrodolasetron decreased by about 27% when dolasetron mesylate was administered intravenously concomitantly with atenolol. ANZEMET did not influence anesthesia recovery time in patients.
- Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs).
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects Pregnancy Category B
- Teratology studies have not revealed evidence of impaired fertility or harm to the fetus due to dolasetron mesylate. These studies have been performed in pregnant rats at intravenous doses up to 60 mg/kg/day (5.4 times the recommended human dose based on body surface area) and pregnant rabbits at intravenous doses up to 20 mg/kg/day (3.2 times the recommended human dose based on body surface area). There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dolasetron (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dolasetron (injection) during labor and delivery.
Nursing Mothers
- It is not known whether dolasetron mesylate is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ANZEMET Injection is administered to a nursing woman.
Pediatric Use
Prevention of chemotherapy-induced nausea and vomiting (CINV)
- Dolasetron is contraindicated in pediatric patients for the prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy.
Prevention and treatment of post-operative nausea and vomiting (PONV)
- Safety and effectiveness in pediatric patients (2 years and older) for prevention and treatment of postoperative nausea and vomiting is based on pharmacokinetic studies and efficacy data in adults. Safety and effectiveness in pediatric patients under 2 years of age have not been established.
Two open-label, noncomparative pharmacokinetic studies have been performed in a total of 30 pediatric patients undergoing surgery with general anesthesia. These patients received ANZEMET Injection either intravenously or orally in juice. Pediatric patients from 2 to 12 years of age participated in these trials, which included an intravenous ANZEMET Injection dose of 1.2 mg/kg, and an oral dose of 1.2 mg/kg. There is no experience in pediatric patients under 2 years of age. Overall, ANZEMET Injection was well tolerated in these pediatric patients. No efficacy information was collected in the pediatric postoperative nausea and vomiting studies.
Geriatic Use
Prevention of chemotherapy-induced nausea and vomiting (CINV)
- Dolasetron is contraindicated in geriatric patients for prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy.
Prevention and treatment of post-operative nausea and vomiting (PONV)
- Controlled clinical studies in the prevention and treatment of post-operative nausea and vomiting did not include sufficient numbers of patients aged 65 years or older – only 57 (2%) geriatric patients (43 received intravenous ANZEMET Injection) out of 3289 total patients participated in the controlled PONV trials – to determine whether they respond differently from younger patients. Other reported clinical experiences have not identified differences in responses between geriatric and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- Elderly patients are at particular risk for prolongation of the PR, QRS, and QT interval; therefore, caution should be exercised and ECG monitoring should be performed when using ANZEMET for prevention of postoperative nausea and vomiting in this population.
- The pharmacokinetics, including clearance of intravenous ANZEMET Injection, in elderly and younger patients are similar. Dosage adjustment is not needed in patients over the age of 65.
Gender
There is no FDA guidance on the use of Dolasetron (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dolasetron (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dolasetron (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dolasetron (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dolasetron (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dolasetron (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Dolasetron (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dolasetron (injection) in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Dolasetron (injection) in the drug label.
Pharmacology
There is limited information regarding Dolasetron (injection) Pharmacology in the drug label.
Mechanism of Action
Structure
- ANZEMET (dolasetron mesylate) is an antinauseant and antiemetic agent. Chemically, dolasetron mesylate is (2α,6α,8α,9aβ)-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl-1H-indole-3-carboxylate monomethanesulfonate, monohydrate. It is a highly specific and selective serotonin subtype 3 (5-HT3) receptor antagonist both in vitro and in vivo. Dolasetron mesylate has the following structural formula:

- The empirical formula is C19H20N2O3 • CH3SO3H • H2O, with a molecular weight of 438.50. Approximately 74% of dolasetron mesylate monohydrate is dolasetron base.
- Dolasetron mesylate monohydrate is a white to off-white powder that is freely soluble in water and propylene glycol, slightly soluble in ethanol, and slightly soluble in normal saline.
- ANZEMET Injection is a clear, colorless, nonpyrogenic, sterile solution for intravenous administration. Each milliliter of ANZEMET Injection contains 20 mg of dolasetron mesylate and 38.2 mg mannitol, USP, with an acetate buffer in water for injection. The pH of the resulting solution is 3.2 to 3.8.
- ANZEMET Injection multidose vials contain a clear, colorless, nonpyrogenic, sterile solution for intravenous administration. Each ANZEMET multidose vial contains 25 mL (500 mg) dolasetron mesylate. Each milliliter contains 20 mg dolasetron mesylate, 29 mg mannitol, USP, and 5 mg phenol, USP, with an acetate buffer in water for injection. The pH of the resulting solution is 3.2 to 3.7.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dolasetron (injection) in the drug label.
Pharmacokinetics
There is limited information regarding Dolasetron (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 24-month carcinogenicity study, there was a statistically significant (P<0.001) increase in the incidence of combined hepatocellular adenomas and carcinomas in male mice treated with 150 mg/kg/day and above. In this study, mice (CD-1) were treated orally with dolasetron mesylate 75, 150 or 300 mg/kg/day (225, 450 or 900 mg/m2/day). For a 50 kg person of average height (1.46 m2 body surface area), these doses represent 3.4, 6.8 and 13.5 times the recommended clinical dose (66.6 mg/m2, intravenous) on a body surface area basis. No increase in liver tumors was observed at a dose of 75 mg/kg/day in male mice and at doses up to 300 mg/kg/day in female mice.
- In a 24-month rat (Sprague-Dawley) carcinogenicity study, oral dolasetron mesylate was not tumorigenic at doses up to 150 mg/kg/day (900 mg/m2/day, 13.5 times the recommended human dose based on body surface area) in male rats and 300 mg/kg/day (1800 mg/m2/day, 27 times the recommended human dose based on body surface area) in female rats.
- Dolasetron mesylate was not genotoxic in the Ames test, the rat lymphocyte chromosomal aberration test, the Chinese hamster ovary (CHO) cell (HGPRT) forward mutation test, the rat hepatocyte unscheduled DNA synthesis (UDS) test or the mouse micronucleus test.
- Dolasetron mesylate was found to have no effect on fertility and reproductive performance at oral doses up to 100 mg/kg/day (600 mg/m2/day, 9 times the recommended human dose based on body surface area) in female rats and up to 400 mg/kg/day (2400 mg/m2/day, 36 times the recommended human dose based on body surface area) in male rats.
Clinical Studies
Prevention of Postoperative Nausea and Vomiting
- ANZEMET Injection administered intravenously at a dose of 12.5 mg approximately 15 minutes before the cessation of general balanced anesthesia (short-acting barbiturate, nitrous oxide, narcotic and analgesic, and skeletal muscle relaxant) was significantly more effective than placebo in preventing postoperative nausea and vomiting. No increased efficacy was seen with higher doses.
- One trial compared single intravenous ANZEMET Injection doses of 12.5, 25, 50, and 100 mg with placebo in 635 women surgical patients undergoing laparoscopic procedures. ANZEMET Injection at a dose of 12.5 mg was statistically superior to placebo for complete response (no vomiting, no rescue medication) (p=.0003). Complete response rates were 50% and 31%, respectively.
- Another trial compared single intravenous ANZEMET Injection doses of 12.5, 25, 50, and 100 mg with placebo in 1030 (722 women and 308 men) surgical patients. In women, the 12.5 mg dose was statistically superior to placebo for complete response. The complete response rates were 50% and 40%, respectively. However, in men, there was no statistically significant difference in complete response between any ANZEMET dose and placebo.
Treatment of Postoperative Nausea and/or Vomiting
- Two randomized, double-blinded trials compared single intravenous ANZEMET Injection doses of 12.5, 25, 50, and 100 mg with placebo in 124 male and 833 female patients who had undergone surgery with general balanced anesthesia and presented with early postoperative nausea or vomiting requiring antiemetic treatment.
- In both studies, the 12.5 mg intravenous dose of ANZEMET was statistically superior to placebo for complete response (no vomiting, no escape medication). No significant increased efficacy was seen with higher doses.
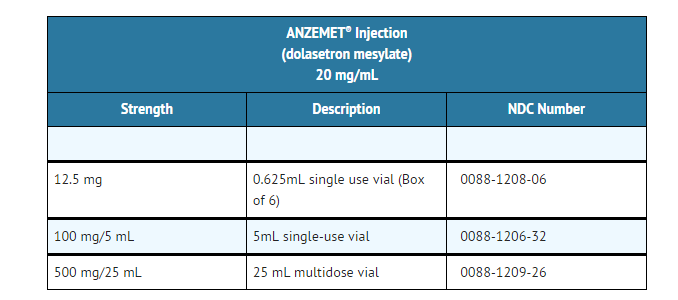
How Supplied
ANZEMET Injection (dolasetron mesylate) is supplied as a clear, colorless solution in single and multidose vials.
Storage
- Store at 20–25°C (68–77°F) with excursions permitted to 15–30°C (59–86°F). Protect from light.
Images
Drug Images
{{#ask: Page Name::Dolasetron (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dolasetron (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dolasetron (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Dolasetron (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Dolasetron (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Dolasetron (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.