Dofetilide: Difference between revisions
Gerald Chi (talk | contribs) m (Changed protection level for "Dofetilide" ([Edit=Allow only autoconfirmed users] (expires 16:54, 24 March 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 16:54, 24 March 2014 (UTC)))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{SS}} | |||
|genericName=Dofetilide | |||
|drugClass=Antiarrhythmic | |||
|indication=Maintenance of Normal Sinus Rhythm (Delay in AF/AFl Recurrence), Conversion of Atrial Fibrillation/Flutter | |||
|hasBlackBoxWarning=Yes | |||
|adverseReactions=chest pain, dizziness , headache | |||
|blackBoxWarningBody=To minimize the risk of induced arrhythmia, patients initiated or re-initiated on TIKOSYN should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation. For detailed instructions regarding dose selection, see DOSAGE AND ADMINISTRATION. TIKOSYN is available only to hospitals and prescribers who have received appropriate TIKOSYN dosing and treatment initiation education; see DOSAGE AND ADMINISTRATION. | |||
|fdaLIADAdult=<h4>Condition 1</h4> | |||
* Dosing Information | |||
:: (Dosage) | |||
}} | |||
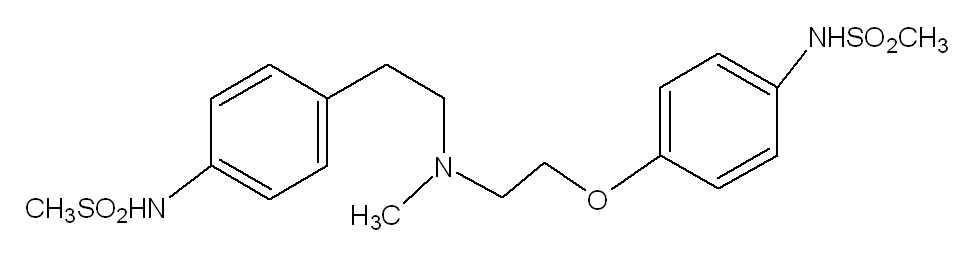
{{drugbox | IUPAC_name = N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methyl-amino] ethyl]phenyl] methanesulfonamide | image = Dofetilide.png | CAS_number = 115256-11-6 | ATC_prefix = C01 | ATC_suffix = BD04 | ATC_supplemental = | PubChem = 71329 | DrugBank = APRD00367 | C=19 | H=27 | N=3 | O=5 | S=2 | molecular_weight = 441.567 g/mol | bioavailability = 96% (oral) | protein_bound = 60% -70% | metabolism = | elimination_half-life = 10 hours | pregnancy_category = | legal_status = | routes_of_administration = }} | {{drugbox | IUPAC_name = N-[4-[2-[2-[4-(methanesulfonamido)phenoxy]ethyl-methyl-amino] ethyl]phenyl] methanesulfonamide | image = Dofetilide.png | CAS_number = 115256-11-6 | ATC_prefix = C01 | ATC_suffix = BD04 | ATC_supplemental = | PubChem = 71329 | DrugBank = APRD00367 | C=19 | H=27 | N=3 | O=5 | S=2 | molecular_weight = 441.567 g/mol | bioavailability = 96% (oral) | protein_bound = 60% -70% | metabolism = | elimination_half-life = 10 hours | pregnancy_category = | legal_status = | routes_of_administration = }} | ||
{{SI}} | {{SI}} | ||
Revision as of 17:24, 14 April 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
{{{blackBoxWarningTitle}}}
See full prescribing information for complete Boxed Warning.
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on TIKOSYN should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation. For detailed instructions regarding dose selection, see DOSAGE AND ADMINISTRATION. TIKOSYN is available only to hospitals and prescribers who have received appropriate TIKOSYN dosing and treatment initiation education; see DOSAGE AND ADMINISTRATION.
|
Overview
Dofetilide is {{{aOrAn}}} Antiarrhythmic that is FDA approved for the {{{indicationType}}} of Maintenance of Normal Sinus Rhythm (Delay in AF/AFl Recurrence), Conversion of Atrial Fibrillation/Flutter. There is a Black Box Warning for this drug as shown here. Common adverse reactions include chest pain, dizziness , headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
<h4>Condition 1</h4>
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Dofetilide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
There is limited information regarding Dofetilide Contraindications in the drug label.
Warnings
|
{{{blackBoxWarningTitle}}}
See full prescribing information for complete Boxed Warning.
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on TIKOSYN should be placed for a minimum of 3 days in a facility that can provide calculations of creatinine clearance, continuous electrocardiographic monitoring, and cardiac resuscitation. For detailed instructions regarding dose selection, see DOSAGE AND ADMINISTRATION. TIKOSYN is available only to hospitals and prescribers who have received appropriate TIKOSYN dosing and treatment initiation education; see DOSAGE AND ADMINISTRATION.
|
There is limited information regarding Dofetilide Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Dofetilide Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Dofetilide Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Dofetilide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Dofetilide in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dofetilide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dofetilide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Dofetilide in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Dofetilide in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Dofetilide in geriatric settings.
Gender
There is no FDA guidance on the use of Dofetilide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dofetilide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dofetilide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dofetilide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dofetilide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dofetilide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Dofetilide Administration in the drug label.
Monitoring
There is limited information regarding Dofetilide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Dofetilide and IV administrations.
Overdosage
There is limited information regarding Dofetilide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Dofetilide Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Dofetilide Mechanism of Action in the drug label.
Structure
There is limited information regarding Dofetilide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Dofetilide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Dofetilide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Dofetilide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Dofetilide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Dofetilide How Supplied in the drug label.
Storage
There is limited information regarding Dofetilide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Dofetilide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dofetilide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Dofetilide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Dofetilide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Dofetilide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Dofetilide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 96% (oral) |
| Protein binding | 60% -70% |
| Elimination half-life | 10 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H27N3O5S2 |
| Molar mass | 441.567 g/mol |
|
WikiDoc Resources for Dofetilide |
|
Articles |
|---|
|
Most recent articles on Dofetilide |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dofetilide at Clinical Trials.gov Clinical Trials on Dofetilide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dofetilide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Dofetilide Discussion groups on Dofetilide Patient Handouts on Dofetilide Directions to Hospitals Treating Dofetilide Risk calculators and risk factors for Dofetilide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Dofetilide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
For patient information, click here
Dofetilide is a class III antiarrhythmic agent that is approved by the Food and Drug Administration (FDA) for the maintenance of sinus rhythm in individuals prone to the formation of atrial fibrillation and flutter, and for the chemical cardioversion to sinus rhythm from atrial fibrillation and flutter.
The chemical name for dofetilide is N-[4-(2-{2-[4-(methanesulphonamido) phenoxyl]-N-methylethylamino}ethyl)phenyl]- methanesulphonamide. It is marketed under the trade name Tikosyn® by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 µg of dofetilide. Due to the pro-arrhythmic potential of dofetilide, it is only available by prescription by physicians who have undergone specific training in the risks of treatment with dofetilide. In addition, it is only available by mail order or through specially trained local pharmacies to individuals who are prescribed dofetilide by a physician who is registered as being able to prescribe the pharmaceutical.
The elimination half-life of dofetilide is roughly 10 hours, however this is variable based on many physiologic factors (most significantly creatinine clearance), and ranges from 4.8 to 13.5 hours.
Mechanism of action
Dofetilide works by selectively blocking the rapid component of the delayed rectifier outward potassium current (IKr).
This causes prolongation of the effective refractory period of accessory pathways (both anterograde and retrograde conduction in the accessory pathway). It is this selective action on accessory pathways that makes dofetilide effective in the treatment of atrial fibrillation and flutter.
Dofetilide does not effect Vmax (The slope of the upstroke of phase 0 depolarization), conduction velocity, or the resting membrane potential.
There is a dose-dependent increase in the QT interval and the corrected QT interval (QTc). Because of this, many practitioners will initiate dofetilide therapy only on individuals under telemetry monitoring or if serial EKG measurements of QT and QTc can be performed.
Metabolism
A steady-state plasma level of dofetilide is achieved in 2-3 days.
80% of dofetilide is excreted by the kidneys, so the dose of dofetilide should be adjusted in individuals with renal insufficiency, based on creatinine clearance.
In the kidneys, dofetilide is eliminated via cation exchange (secretion). Agents that interfere with the renal cation exchange system, such as verapamil, cimetidine, hydrochlorothiazide, itraconazole, ketoconazole, prochlorperazine, and trimethoprim should not be administered to individuals taking dofetilide.
About 20 percent of dofetilide is metabolized in the liver via the CYP3A4 isoenzyme of the cytochrome P450 enzyme system. Drugs that interfere with the activity of the CYP3A4 isoenzyme can increase serum dofetilide levels. If the renal cation exchange system is interfered with (as with the medications listed above), a larger percentage of dofetilide is cleared via the CYP3A4 isoenzyme system.
Side effects
Torsades de pointes is the most serious side effect of dofetilide therapy. The incidence of torsades de pointes is dose-related, and is 0.3-10.5%. The risk appears to be dose-dependent, with an increased incidence of torsades de pointes associated with higher doses of dofetilide administered.
The risk of inducing torsades de pointes can be decreased by taking precautions when initiating therapy, such as hospitalizing individuals for a minimum of three days for serial creatinine measurement, continuous telemetry monitoring and availability of cardiac resuscitation.
Clinical use
Based on the results of the Danish Investigations of Arrhythmias and Mortality on Dofetilide (DIAMOND) study, dofetilide does not affect mortality in the treatment of patients post-myocardial infarction with left ventricular dysfunction.3 Because of the results of the DIAMOND study, many physicians use dofetilide in the suppression of atrial fibrillation in individuals with LV dysfunction.
See also
References
- Thomas L. Lenz, Pharm.D., and Daniel E. Hilleman, Pharm.D., Department of Cardiology, Creighton University, Omaha, Nebraska. Dofetilide, a New Class III Antiarrhythmic Agent. Pharmacotherapy 20(7):776-786, 2000. (Medline abstract)
- Lenz TL, Hilleman DE. Dofetilide: A new antiarrhythmic agent approved for conversion and/or maintenance of atrial fibrillation/atrial flutter. Drugs Today (Barc). 2000 Nov;36(11):759-71. (Medline abstract)
- Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999 Sep 16;341(12):857-65. (Medline abstract)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Antiarrhythmic agents
- Drugs