Diethyl ether: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
Kiran Singh (talk | contribs) No edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox | {{Chembox | ||
| | | Watchedfields = changed | ||
| | | verifiedrevid = 477163372 | ||
| | | Name = Diethyl ether | ||
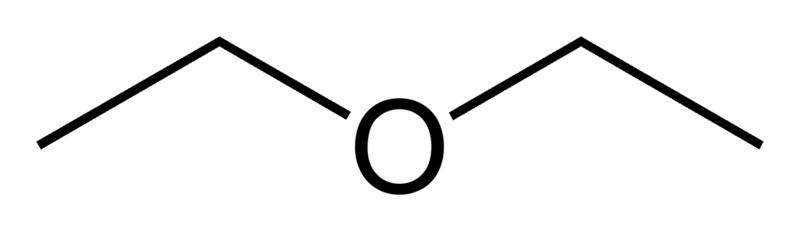
| | | ImageFile1 = Diethyl-ether-2D-skeletal.png | ||
| | | ImageSize1 = | ||
| | | ImageName1 = Skeletal formula | ||
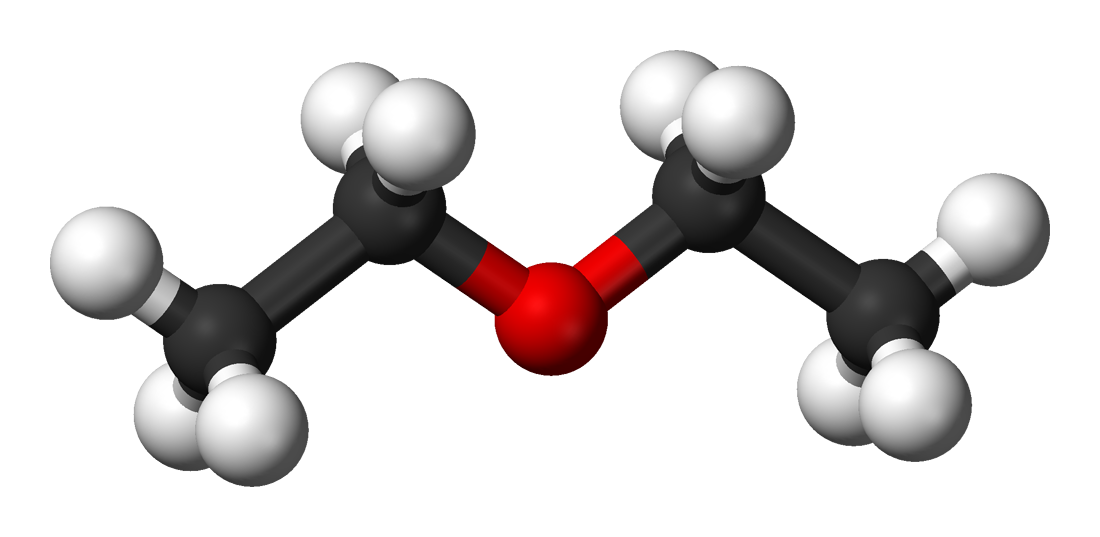
| | | ImageFile2 = File:Diethyl-ether-3D-balls.png | ||
| | | ImageSize2 = | ||
| | | ImageName2 = Ball-and-stick model | ||
| Section1 = {{Chembox Identifiers | | ImageFile3 = Diethyl_Ether_Electron_Rendering.png | ||
| | | ImageSize3 = | ||
| | | ImageName3 = Diethyl Ether Electron Rendering | ||
| | | IUPACName = Ethoxyethane | ||
| OtherNames = Diethyl ether; Dether; Ethyl ether; Ethyl oxide; 3-Oxapentane; Ethoxyethane; Diethyl oxide; Solvent ether | |||
|Section1={{Chembox Identifiers | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 35702 | |||
| SMILES = CCOCC | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 0F5N573A2Y | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D01772 | |||
| InChI = 1/C4H10O/c1-3-5-4-2/h3-4H2,1-2H3 | |||
| InChIKey = RTZKZFJDLAIYFH-UHFFFAOYAB | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 16264 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C4H10O/c1-3-5-4-2/h3-4H2,1-2H3 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = RTZKZFJDLAIYFH-UHFFFAOYSA-N | |||
| CASNo = 60-29-7 | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3168 | |||
| PubChem = 3283 | |||
| RTECS = KI5775000 | |||
| ATCCode_prefix = N01 | |||
| ATCCode_suffix = AA01 | |||
}} | }} | ||
| Section2 = {{Chembox Properties | |Section2={{Chembox Properties | ||
| | | C =4|H=10|O=1 | ||
| | | Appearance = Colorless liquid | ||
| | | Odor = pungent, sweetish odor<ref name=PGCH/> | ||
| | | Density = 0.7134 g/cm<sup>3</sup>, liquid | ||
| | | Solubility = 69 g/L (20 °C) | ||
| | | MeltingPtC = −116.3 | ||
| | | Melting_notes = | ||
| | | BoilingPtC = 34.6 | ||
| | | Boiling_notes = | ||
| | | RefractIndex = 1.353 (20 °C) | ||
| pKa = | |||
| pKb = | |||
| Viscosity = 0.224 [[Poise|cP]] (25 °C) | |||
| VaporPressure = 440 mmHg (20°C)<ref name=PGCH/> | |||
}} | }} | ||
| Section3 = {{Chembox Structure | |Section3={{Chembox Structure | ||
| | | MolShape = | ||
| | | Dipole = 1.15 [[Debye|D]] (gas) | ||
}} | }} | ||
| | |Section4={{Chembox Thermochemistry | ||
| | | DeltaHc = -2732.1 ± 1.9 kJ/mol | ||
| | | DeltaHf = -271.2 ± 1.9 kJ/mol | ||
| Entropy = 253.5 J/mol·K | |||
| HeatCapacity = 172.5 J/mol·K | |||
| | |||
| | |||
}} | }} | ||
| Section8 = {{Chembox Related | |Section7={{Chembox Hazards | ||
| | | ExternalMSDS = [http://www.chem.purdue.edu/chemsafety/safetyclass/SDS/GHS-Et2O.pdf External MSDS] | ||
| | | MainHazards = Extremely Flammable, harmful to skin, decomposes to explosive peroxides in air and light<ref name=PGCH/> | ||
| NFPA-H = 2 | |||
| NFPA-F = 4 | |||
| NFPA-R = 1 | |||
| AutoignitionPtC = 160 | |||
| Autoignition_ref = <ref name="MSDS">{{cite web | url = http://hazard.com/msds/mf/baker/baker/files/e2340.htm | title = Ethyl Ether MSDS | publisher = J.T. Baker | accessdate = 2010-06-24}}</ref> | |||
| FlashPtC = −45 | |||
| Flash_notes = <ref name="MSDS"/> | |||
| ExploLimits = 1.9-48.0% <ref name = "Yaws">Carl L. Yaws, Chemical Properties Handbook, McGraw-Hill, New York, 1999, page 567</ref> | |||
| RPhrases = {{R12}} {{R19}} {{R20/22}} {{R66}} {{R67}} | |||
| SPhrases = {{S9}} {{S16}} {{S29}} {{S33}} | |||
| PEL = TWA 400 ppm (1200 mg/m<sup>3</sup>)<ref name=PGCH>{{PGCH|0277}}</ref> | |||
| IDLH = 1900 ppm<ref name=PGCH/> | |||
| REL = No established REL<ref name=PGCH/> | |||
}} | |||
|Section8={{Chembox Related | |||
| Function = [[Ether]]s | |||
| OtherFunctn = [[Dimethyl ether]]<br>[[Methoxypropane]] | |||
| OtherCpds = [[Diethyl sulfide]]<br>[[Butanol]]s ([[isomer]]) | |||
}} | }} | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Diethyl ether''', also known as '''ethoxyethane''', '''ethyl ether''', '''sulfuric ether''', or simply '''ether''', is an [[organic compound]] in the [[ether]] class with the formula {{chem|({{chem|C|2|H|5}})|2|O}}. It is a colorless, highly [[Volatility (chemistry)|volatile]] [[flammable liquid]]. It is commonly used as a [[solvent]] and was once used as a [[general anesthetic]]. It has narcotic properties and has been known to cause temporary dependence, the only symptom of which is the will to consume more, sometimes referred to as [[etheromania]]. | |||
== | == History == | ||
The compound may have been created by either [[Jābir ibn Hayyān]] in the 8th century<ref name=Barash>{{cite book |author=Toski, Judith A; Bacon, Douglas R; Calverley, Rod K |title=The history of Anesthesiology |edition=4 |publisher=Lippincott Williams & Wilkins |year=2001 |isbn=978-0-7817-2268-1 |page=3 |work=In: Barash, Paul G; Cullen, Bruce F; Stoelting, Robert K. Clinical Anesthesia}}</ref> or [[Ramon Llull]] in 1275,<ref name=Barash/><ref name=Lullus>{{cite book |author=Hademenos, George J.; Murphree, Shaun; Zahler, Kathy; Warner, Jennifer M. |title=McGraw-Hill's PCAT |publisher=McGraw-Hill |page=39 |url=http://books.google.com/?id=8MwxkLP87IUC&pg=PA39&lpg=PA39&dq=Raymundus+Lullus+ether#v=onepage&q=Raymundus%20Lullus%20ether&f=false |accessdate=2011-05-25 |isbn=978-0-07-160045-3 |date=2008-11-12}}</ref> although there is no contemporary evidence of this. It was first synthesized in 1540 by [[Valerius Cordus]], who called it "sweet oil of vitriol" (''oleum dulce vitrioli'') — the name reflects the fact that it is obtained by distilling a mixture of [[ethanol]] and [[sulfuric acid]] (then known as oil of vitriol) — and noted some of its [[medicinal properties]].<ref name=Barash/> At about the same time, [[Paracelsus]] discovered ether's [[analgesic]] properties in chickens.<ref name=Barash/> The name ''ether'' was given to the substance in 1729 by [[August Sigmund Frobenius]].<ref>Dr. Frobenius (1729) [http://rstl.royalsocietypublishing.org/content/36/407-416/283.full.pdf+html "An account of a spiritus vini æthereus, together with several experiments tried therewith,"] ''Philosophical Transactions of the Royal Society'' (London), '''36''' : 283-289.</ref> | |||
== Applications == | |||
It is particularly important as a solvent in the production of cellulose plastics such as [[cellulose acetate]].<ref name="kirk">{{cite encyclopedia | |||
| title =Ethers, by Lawrence Karas and W. J. Piel | |||
| encyclopedia =Kirk‑Othmer Encyclopedia of Chemical Technology | |||
| volume = | |||
| pages = | |||
| publisher =John Wiley & Sons, Inc. | |||
| year =2004 | |||
| id =}}</ref> | |||
== | === As a fuel === | ||
Diethyl ether has a high [[cetane number]] of 85-96 and is used as a [[starting fluid]], in combination with petroleum distillates for gasoline and diesel engines<ref>{{cite web|url=http://www.valvoline.com/pages/products/product_detail.asp?product=38§ion=402 | title = Extra Strength Starting Fluid: How it Works | publisher = Valvovine | accessdate=2007-09-05 |archiveurl = http://web.archive.org/web/20070927222427/http://www.valvoline.com/pages/products/product_detail.asp?product=38§ion=402 <!-- Bot retrieved archive --> |archivedate = 2007-09-27}}</ref> because of its high volatility and low [[flash point]]. For the same reason it is also used as a component of the fuel mixture for [[carbureted compression ignition model engine]]s. In this way diethyl ether is very similar to one of its precursors, [[ethanol]]. | |||
Diethyl ether | === Laboratory uses === | ||
Diethyl ether is a common laboratory [[solvent]]. It has limited [[solubility]] in [[Water (molecule)|water]] (6.05 g/100 mL at 25 °C.) <ref>The Merck Index, 10th Edition, Martha Windholz, editor, Merck & Co., Inc, Rahway, NJ, 1983, page 551</ref> and dissolves 1.5 g/100 mL water at 25 °C.<ref name="water_in_ether">{{cite journal | |||
| author = H. H. Rowley, Wm. R. Reed | |||
| year = 1951 | |||
| title = Solubility of Water in Diethyl Ether at 25 ° | |||
| journal = J. Am. Chem. Soc. | |||
| volume = 73 | |||
| issue = 6 | |||
| pages = 2960–2960 | |||
| pmid = | |||
| doi = 10.1021/ja01150a531 | |||
| url = http://pubs.acs.org/doi/abs/10.1021/ja01150a531 | |||
}}</ref> Therefore, it is commonly used for [[liquid-liquid extraction]]. When used with an aqueous solution, the organic layer is on top as the diethyl ether has a lower density than the water. Please note that this is not an effective means of extracting diethyl ether as water is highly [[chemical polarity|polar]] while ether is non-polar. It is also a common solvent for the [[Grignard reaction]] in addition to other reactions involving organometallic reagents. Due to its application in the manufacturing of illicit substances, it is listed in the Table II precursor under the [[United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances]] as well as substances such as [[acetone]], [[toluene]] and [[sulphuric acid]].<ref>[http://www.incb.org/pdf/e/list/red.pdf Microsoft Word - RedListE2007.doc<!-- Bot generated title -->]</ref> | |||
== | === Anesthetic use === | ||
[[Image:Ether monument-Boston.JPG||thumb|300px|left|Panel from monument in Boston commemorating Morton's demonstration of ether's anesthetic use.]] | |||
[[William T.G. Morton]] participated in a public demonstration of ether anesthesia on October 16, 1846 at the [[Ether Dome]] in [[Boston, Massachusetts]]. However, [[Crawford Williamson Long]], M.D., is now known to have demonstrated its use privately as a [[general anesthetic]] in surgery to officials in Georgia, as early as March 30, 1842, and Long publicly demonstrated ether's use as a surgical anesthetic on six occasions before the Boston demonstration.<ref name=hill>Hill, John W. and Kolb, Doris K. ''Chemistry for changing times: 10th edition''. Page 257. Pearson: Prentice Hall. Upper saddle river, New Jersey. 2004.</ref><ref name="newgeorgiaencylcopedia">{{cite web | url=http://www.georgiaencyclopedia.org/articles/science-medicine/crawford-long-1815-1878 | title=Crawford Long (1815-1878) | publisher=University of Georgia Press | work=New Georgia Encylcopedia | date=May 14, 2004 | accessdate=February 13, 2015 | author=Madden, M. Leslie}}</ref><ref name="southernmedicalassoc">{{cite web | url=http://sma.org/sma-alliance/doctors-day-2/crawford-w-long/ | title=Crawford W. Long | publisher=Southern Medical Association | work=Doctors' Day | accessdate=February 13, 2015}}</ref> British doctors were aware of the anesthetic properties of ether as early as 1840 where it was widely prescribed in conjunction with opium.<ref name= Grattan>Grattan, N. ''Treatment of Uterine Haemorrhage''. Provincial Medicine and Surgical Journal. Vol. 1, No. 6 (Nov. 7, 1840), p. 107.</ref> | |||
Diethyl ether largely supplanted the use of [[chloroform]] as a general anesthetic due to ethers more favorable [[therapeutic index]], that is, a greater difference between an effective dose and a potentially toxic dose.<ref>{{cite journal | author = Calderone, F.A. | journal = J. Pharmacology Experimental Therapeutics | year = 1935 | volume = 55 | issue= 1 | pages = 24–39 | url = http://jpet.aspetjournals.org/cgi/reprint/55/1/24.pdf Jpet.aspetjournals.org}}</ref> Because of its associations with Boston, the use of ether became known as the "Yankee Dodge." | |||
== | Diethyl ether depresses the [[myocardium]] and increases tracheobronchial secretions.<ref>{{cite journal |title=Ether and its effects in Anesthesia |url=http://anesthesiageneral.com/ether-effects/}}</ref> | ||
Diethyl ether could also be mixed with other anesthetic agents such as [[chloroform]] to make [[C.E. mixture]], or chloroform and [[Ethanol|alcohol]] to make [[A.C.E. mixture]]. | |||
Today, ether is rarely used. The use of flammable ether | Today, ether is rarely used. The use of flammable ether was displaced by nonflammable fluorinated hydrocarbon anesthetics. [[Halothane]] was the first such anesthetic developed and other currently used inhaled anesthetics, such as isoflurane, desflurane, and sevoflurane, are halogenated ethers.<ref name=morgan>Morgan, G. Edward, Jr. et al. "Clinical Anesthesiology" 3rd Ed, p. 3. New York: Mc Graw-Hill. 2002</ref> Diethyl ether was found to have undesirable side effects, such as post-anesthetic nausea and vomiting. Modern anesthetic agents reduce these side effects.<ref name=hill/> | ||
=== Medical use === | |||
It was once used in pharmaceuticals. A mixture of alcohol and ether was known as "Spirit of ether" or Hoffman's Drops. In the United States, it was removed from [[United States Pharmacopeia|Pharmacopeia]] prior to June 1917.<ref>The National druggist, Volume 47, June 1917, pp.220</ref> | |||
===Recreational use=== | === Recreational use === | ||
{{See also|Addiction to ether consumption}} | |||
The anesthetic effects of ether have made it a recreational drug. Diethyl ether in anesthetic dosage is an inhalant which has a long history of recreational use. One disadvantage is the high flammability, especially in conjunction with oxygen. One advantage is a well-defined margin between therapeutic and toxic doses, which means one would lose consciousness before dangerous levels of dissolved ether in blood would be reached. With a strong, dense smell, ether causes irritation to respiratory mucosa and is uncomfortable to breathe, and in overdose triggering salivation, vomiting, coughing or spasms. In concentrations of 3-5% in air, an anesthetic effect can slowly be achieved in 15–20 minutes of breathing approximately 15-20ml of ether, depending on body weight and physical condition. Ether causes a very long excitation stage prior to blacking out. | |||
In the 19th century and early 20th century ether drinking was popular among Polish peasants.<ref>{{Cite journal | |||
| last = Zandberg | first = Adrian | |||
| title = Short Article "Villages … Reek of Ether Vapours": Ether Drinking in Silesia before 1939 | |||
| journal = Medical History | |||
| volume = 54 | |||
| issue = 3 | |||
| pages = 387–396 | |||
| year = 2010 | |||
| pmid = 20592886 | |||
| pmc = 2890321 | |||
| doi =10.1017/s002572730000466x}}</ref> It is a traditional and still relatively popular recreational drug among [[Lemkos]].<ref>{{Cite web | |||
| url = http://histmag.org/?id=349 | |||
| title = Łemkowska Watra w Żdyni 2006 – pilnowanie ognia pamięci | |||
| last = Kaszycki | |||
| first = Nestor | |||
| date = 2006-08-30 | |||
| work = Histmag.org – historia od podszewki | |||
| publisher = i-Press | |||
| location = Kraków, Poland | |||
| language = Polish | |||
| accessdate = 2009-11-25 | |||
| quote = Dawniej eteru używało się w lecznictwie do narkozy, ponieważ ma właściwości halucynogenne, a już kilka kropel inhalacji wystarczyło do silnego znieczulenia pacjenta. Jednak eter, jak każda ciecz, może teoretycznie być napojem. Łemkowie tę teorię praktykują. Mimo to, nazywanie skroplonego eteru – „kropki” – ich „napojem narodowym” byłoby przesadą. Chociaż stanowi to pewną część mitu „bycia Łemkiem”. | |||
}}</ref> It is usually consumed in a small quantity (''[[wikt:kropka|kropka]]'', or "dot") poured over [[milk]], water with sugar or [[orange juice]] in a [[shot glass]]. | |||
== Metabolism == | |||
A [[cytochrome P450]] enzyme is proposed to metabolize diethyl ether.<ref>[http://www.fgsc.net/asilomar1997/secmetab.html 109. Aspergillus flavus mutant strain 241, blocked in aflatoxin biosynthesis, does not accumulate aflR transcript.] Matthew P. Brown and Gary A. Payne, [[North Carolina State University]], Raleigh, NC 27695 fgsc.net</ref> | |||
Diethyl ether inhibits [[alcohol dehydrogenase]], and thus slows the metabolism of [[ethanol]].<ref>{{cite journal | author = P. T. Normann, A. Ripel and J. Morland | title = Diethyl Ether Inhibits Ethanol Metabolism in Vivo by Interaction with Alcohol Dehydrogenase | year = 1987 | journal = [[Alcoholism: Clinical and Experimental Research]] | volume = 11 | issue = 2 | pages = 163–166 | doi = 10.1111/j.1530-0277.1987.tb01282.x | pmid=3296835}}</ref> It also inhibits metabolism of other drugs requiring [[oxidative metabolism]]. | |||
For example [[diazepam]] requires hepatic oxidization whereas its oxidized metabolite [[oxazepam]] does not.<ref>{{cite journal | title = Inhibition of N-Nitrosodimethylamine Metabolism in Rats by Ether Anesthesia | author = Larry K. Keefer, William A. Garland, Neil F. Oldfield, James E. Swagzdis, and Bruce A. Mico | journal = [[Cancer Research (journal)|Cancer Research]] | volume = 45 | pages = 5457–60 | year = 1985 | url = http://cancerres.aacrjournals.org/cgi/reprint/45/11_Part_1/5457.pdf | pmid=4053020 | issue = 11 Pt 1}}</ref> | |||
==Production== | == Production == | ||
Most diethyl ether is produced as a byproduct of the vapor-phase [[Hydration reaction|hydration]] of [[ethylene]] to make [[ethanol]]. This process uses solid-supported [[phosphoric acid]] [[Catalysis|catalysts]] and can be adjusted to make more ether if the need arises.<ref name="kirk"/> Vapor-phase [[Dehydration reaction|dehydration]] of ethanol over some [[Aluminium oxide|alumina]] catalysts can give diethyl ether yields of up to 95%.<ref>{{cite book |last= |first= |authorlink= |coauthors= |title=Ethyl Ether, Chem. Economics Handbook |year=1991 |publisher=SRI International |location=Menlo Park, Calif |isbn= }}</ref> | |||
Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. [[Ethanol]] is mixed with a strong | Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.<ref>{{cite book|last=Cohen|first=Julius Berend|title=A Class-book of Organic Chemistry, Volume 1|year=1920|publisher=Macmillan and Co.|location=London|page=39|url=http://books.google.com/books?id=jyZIAAAAIAAJ&pg=PA37&dq=the+structure+of+ethyl+alcohol+cohen+julius+diethyl+ether&hl=en&sa=X&ei=fA2qUselPM_koATuhoCgAw&ved=0CEIQ6AEwAA#v=onepage&q=the%20structure%20of%20ethyl%20alcohol%20cohen%20julius%20diethyl%20ether&f=false}}</ref> [[Ethanol]] is mixed with a strong acid, typically [[sulfuric acid]], H<sub>2</sub>SO<sub>4</sub>. The acid [[Dissociation (chemistry)|dissociates]] in the aqueous environment producing [[hydronium]] ions, H<sub>3</sub>O<sup>+</sup>. A hydrogen ion [[Protonation|protonates]] the [[Electronegativity|electronegative]] oxygen atom of the [[ethanol]], giving the ethanol molecule a positive charge: | ||
:CH<sub>3</sub>CH<sub>2</sub>OH + H<sup>+</sup> → CH<sub>3</sub>CH<sub>2</sub>OH<sub>2</sub><sup>+</sup> | :CH<sub>3</sub>CH<sub>2</sub>OH + H<sub>3</sub>O<sup>+</sup> → CH<sub>3</sub>CH<sub>2</sub>OH<sub>2</sub><sup>+</sup> + H<sub>2</sub>O | ||
A [[nucleophilic]] oxygen atom of unprotonated ethanol [[Single displacement reaction|displaces]] a water molecule from the protonated ([[electrophilic]]) ethanol molecule, producing water, a hydrogen ion and diethyl ether. | A [[nucleophilic]] oxygen atom of unprotonated ethanol [[Single displacement reaction|displaces]] a water molecule from the protonated ([[electrophilic]]) ethanol molecule, producing water, a hydrogen ion and diethyl ether. | ||
: CH<sub>3</sub>CH<sub>2</sub>OH<sub>2</sub><sup>+</sup> + CH<sub>3</sub>CH<sub>2</sub>OH → H<sub>2</sub>O + H<sup>+</sup> + CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub> | |||
This reaction must be carried out at temperatures lower than 150 °C in order to ensure that an elimination product ([[ethylene]]) is not a product of the reaction. At higher temperatures, ethanol will dehydrate to form ethylene. The reaction to make diethyl ether is reversible, so eventually an [[Chemical equilibrium|equilibrium]] between reactants and products is achieved. Getting a good yield of ether requires that ether be distilled out of the reaction mixture before it reverts to ethanol, taking advantage of [[Le Chatelier's principle]]. | |||
The reaction to make diethyl ether is reversible, so eventually an [[Chemical equilibrium|equilibrium]] between reactants and products is achieved. Getting a good yield of ether requires that ether be distilled out of the reaction mixture before it reverts to ethanol, taking advantage of [[Le Chatelier's principle]]. | |||
Another reaction that can be used for the preparation of ethers is the [[Williamson ether synthesis]], in which an [[alkoxide]] (produced by dissolving an [[alkali metal]] in the alcohol to be used) performs a [[nucleophilic substitution]] upon an [[alkyl halide]]. | |||
== Safety and stability == | |||
Diethyl ether is extremely flammable and may be explosive.<ref name=msds01>http://www.chem.purdue.edu/chemsafety/safetyclass/SDS/GHS-Et2O.pdf</ref> | |||
= | Since ether is heavier than air it can collect low to the ground and the vapour may travel considerable distances to ignition sources, which need not be an open flame, but may be a hot plate, steam pipe, heater etc.{{r|msds01}} Vapour may be ignited by the static electricity which can build up when ether is being poured from one vessel into another. The autoignition temperature of diethyl ether is {{convert|160|C|F}}. A common practice in chemical labs is to use steam (thus limiting the temperature to {{convert|100|C|F}} ) when ether must be heated or distilled. The diffusion of diethyl ether in air is 0.918·10<sup>−5</sup> m<sup>2</sup>/s (298K, 101.325 kPa).{{Citation needed|date=August 2011}} | ||
Ether is sensitive to light and air, tending to form explosive [[diethyl ether peroxide|peroxides]].{{r|msds01}} Ether peroxides are higher boiling than ether and are contact explosives when dry.{{r|msds01}} Commercial diethyl ether is typically supplied with trace amounts of the [[antioxidant]] [[butylated hydroxytoluene]] (BHT), which reduces the formation of peroxides. Storage over [[sodium hydroxide]] precipitates the intermediate ether hydroperoxides. Water and peroxides can be removed by either distillation from [[sodium]] and [[benzophenone]], or by passing through a column of [[activated alumina]].<ref>{{cite book | author = W. L. F. Armarego and C. L. L. Chai | title = Purification of laboratory chemicals | year = 2003 | publisher = Butterworth-Heinemann | location = Boston | isbn = 978-0-7506-7571-0}}</ref> | |||
==References== | ==References== | ||
{{ | {{Reflist|2}} | ||
==External links== | == External links == | ||
* [http:// | {{Commons category}} | ||
* [http:// | * [http://journals.lww.com/anesthesiology/Abstract/1992/10000/Michael_Faraday_and_His_Contribution_to_Anesthesia.27.aspx Michael Faraday's announcement of ether as an anesthetic in 1818] | ||
* [http://www. | * Calculation of [http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe?component=Diethyl%20ether vapor pressure], [http://ddbonline.ddbst.de/DIPPR105DensityCalculation/DIPPR105CalculationCGI.exe?component=Diethyl%20ether liquid density], [http://ddbonline.ddbst.de/VogelCalculation/VogelCalculationCGI.exe?component=Diethyl%20ether dynamic liquid viscosity], [http://ddbonline.ddbst.de/DIPPR106SFTCalculation/DIPPR106SFTCalculationCGI.exe?component=Diethyl%20ether surface tension] of diethyl ether, ddbonline.ddbst.de | ||
* [http://www.cdc.gov/niosh/npg/npgd0277.html CDC - NIOSH Pocket Guide to Chemical Hazards] | |||
{{General anesthetics}} | {{General anesthetics}} | ||
{{GABAAR PAMs}} | |||
[[Category:Ethers]] | [[Category:Ethers]] | ||
[[Category: | [[Category:General anesthetics]] | ||
[[Category: | [[Category:Dissociative drugs]] | ||
[[Category:Ether solvents]] | [[Category:Ether solvents]] | ||
[[Category: | [[Category:GABAA receptor positive allosteric modulators]] | ||
[[Category:NMDA receptor antagonists]] | |||
[[Category:Glycine receptor agonists]] | |||
[[ | |||
[[ | |||
Latest revision as of 12:25, 16 April 2015
| |
| |
| Diethyl Ether Electron Rendering | |
| Names | |
|---|---|
| IUPAC name
Ethoxyethane
| |
| Other names
Diethyl ether; Dether; Ethyl ether; Ethyl oxide; 3-Oxapentane; Ethoxyethane; Diethyl oxide; Solvent ether
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H10O | |
| Molar mass | 74.12 g·mol−1 |
| Hazards | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Diethyl ether |
|
Articles |
|---|
|
Most recent articles on Diethyl ether Most cited articles on Diethyl ether |
|
Media |
|
Powerpoint slides on Diethyl ether |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Diethyl ether at Clinical Trials.gov Trial results on Diethyl ether Clinical Trials on Diethyl ether at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Diethyl ether NICE Guidance on Diethyl ether
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Diethyl ether Discussion groups on Diethyl ether Patient Handouts on Diethyl ether Directions to Hospitals Treating Diethyl ether Risk calculators and risk factors for Diethyl ether
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Diethyl ether |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Diethyl ether, also known as ethoxyethane, ethyl ether, sulfuric ether, or simply ether, is an organic compound in the ether class with the formula (C
2H
5)
2O. It is a colorless, highly volatile flammable liquid. It is commonly used as a solvent and was once used as a general anesthetic. It has narcotic properties and has been known to cause temporary dependence, the only symptom of which is the will to consume more, sometimes referred to as etheromania.
History
The compound may have been created by either Jābir ibn Hayyān in the 8th century[4] or Ramon Llull in 1275,[4][5] although there is no contemporary evidence of this. It was first synthesized in 1540 by Valerius Cordus, who called it "sweet oil of vitriol" (oleum dulce vitrioli) — the name reflects the fact that it is obtained by distilling a mixture of ethanol and sulfuric acid (then known as oil of vitriol) — and noted some of its medicinal properties.[4] At about the same time, Paracelsus discovered ether's analgesic properties in chickens.[4] The name ether was given to the substance in 1729 by August Sigmund Frobenius.[6]
Applications
It is particularly important as a solvent in the production of cellulose plastics such as cellulose acetate.[7]
As a fuel
Diethyl ether has a high cetane number of 85-96 and is used as a starting fluid, in combination with petroleum distillates for gasoline and diesel engines[8] because of its high volatility and low flash point. For the same reason it is also used as a component of the fuel mixture for carbureted compression ignition model engines. In this way diethyl ether is very similar to one of its precursors, ethanol.
Laboratory uses
Diethyl ether is a common laboratory solvent. It has limited solubility in water (6.05 g/100 mL at 25 °C.) [9] and dissolves 1.5 g/100 mL water at 25 °C.[10] Therefore, it is commonly used for liquid-liquid extraction. When used with an aqueous solution, the organic layer is on top as the diethyl ether has a lower density than the water. Please note that this is not an effective means of extracting diethyl ether as water is highly polar while ether is non-polar. It is also a common solvent for the Grignard reaction in addition to other reactions involving organometallic reagents. Due to its application in the manufacturing of illicit substances, it is listed in the Table II precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances as well as substances such as acetone, toluene and sulphuric acid.[11]
Anesthetic use

William T.G. Morton participated in a public demonstration of ether anesthesia on October 16, 1846 at the Ether Dome in Boston, Massachusetts. However, Crawford Williamson Long, M.D., is now known to have demonstrated its use privately as a general anesthetic in surgery to officials in Georgia, as early as March 30, 1842, and Long publicly demonstrated ether's use as a surgical anesthetic on six occasions before the Boston demonstration.[12][13][14] British doctors were aware of the anesthetic properties of ether as early as 1840 where it was widely prescribed in conjunction with opium.[15] Diethyl ether largely supplanted the use of chloroform as a general anesthetic due to ethers more favorable therapeutic index, that is, a greater difference between an effective dose and a potentially toxic dose.[16] Because of its associations with Boston, the use of ether became known as the "Yankee Dodge."
Diethyl ether depresses the myocardium and increases tracheobronchial secretions.[17]
Diethyl ether could also be mixed with other anesthetic agents such as chloroform to make C.E. mixture, or chloroform and alcohol to make A.C.E. mixture.
Today, ether is rarely used. The use of flammable ether was displaced by nonflammable fluorinated hydrocarbon anesthetics. Halothane was the first such anesthetic developed and other currently used inhaled anesthetics, such as isoflurane, desflurane, and sevoflurane, are halogenated ethers.[18] Diethyl ether was found to have undesirable side effects, such as post-anesthetic nausea and vomiting. Modern anesthetic agents reduce these side effects.[12]
Medical use
It was once used in pharmaceuticals. A mixture of alcohol and ether was known as "Spirit of ether" or Hoffman's Drops. In the United States, it was removed from Pharmacopeia prior to June 1917.[19]
Recreational use
The anesthetic effects of ether have made it a recreational drug. Diethyl ether in anesthetic dosage is an inhalant which has a long history of recreational use. One disadvantage is the high flammability, especially in conjunction with oxygen. One advantage is a well-defined margin between therapeutic and toxic doses, which means one would lose consciousness before dangerous levels of dissolved ether in blood would be reached. With a strong, dense smell, ether causes irritation to respiratory mucosa and is uncomfortable to breathe, and in overdose triggering salivation, vomiting, coughing or spasms. In concentrations of 3-5% in air, an anesthetic effect can slowly be achieved in 15–20 minutes of breathing approximately 15-20ml of ether, depending on body weight and physical condition. Ether causes a very long excitation stage prior to blacking out.
In the 19th century and early 20th century ether drinking was popular among Polish peasants.[20] It is a traditional and still relatively popular recreational drug among Lemkos.[21] It is usually consumed in a small quantity (kropka, or "dot") poured over milk, water with sugar or orange juice in a shot glass.
Metabolism
A cytochrome P450 enzyme is proposed to metabolize diethyl ether.[22]
Diethyl ether inhibits alcohol dehydrogenase, and thus slows the metabolism of ethanol.[23] It also inhibits metabolism of other drugs requiring oxidative metabolism. For example diazepam requires hepatic oxidization whereas its oxidized metabolite oxazepam does not.[24]
Production
Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises.[7] Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%.[25]
Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis.[26] Ethanol is mixed with a strong acid, typically sulfuric acid, H2SO4. The acid dissociates in the aqueous environment producing hydronium ions, H3O+. A hydrogen ion protonates the electronegative oxygen atom of the ethanol, giving the ethanol molecule a positive charge:
- CH3CH2OH + H3O+ → CH3CH2OH2+ + H2O
A nucleophilic oxygen atom of unprotonated ethanol displaces a water molecule from the protonated (electrophilic) ethanol molecule, producing water, a hydrogen ion and diethyl ether.
- CH3CH2OH2+ + CH3CH2OH → H2O + H+ + CH3CH2OCH2CH3
This reaction must be carried out at temperatures lower than 150 °C in order to ensure that an elimination product (ethylene) is not a product of the reaction. At higher temperatures, ethanol will dehydrate to form ethylene. The reaction to make diethyl ether is reversible, so eventually an equilibrium between reactants and products is achieved. Getting a good yield of ether requires that ether be distilled out of the reaction mixture before it reverts to ethanol, taking advantage of Le Chatelier's principle.
Another reaction that can be used for the preparation of ethers is the Williamson ether synthesis, in which an alkoxide (produced by dissolving an alkali metal in the alcohol to be used) performs a nucleophilic substitution upon an alkyl halide.
Safety and stability
Diethyl ether is extremely flammable and may be explosive.[27]
Since ether is heavier than air it can collect low to the ground and the vapour may travel considerable distances to ignition sources, which need not be an open flame, but may be a hot plate, steam pipe, heater etc.[27] Vapour may be ignited by the static electricity which can build up when ether is being poured from one vessel into another. The autoignition temperature of diethyl ether is 160 °C (320 °F). A common practice in chemical labs is to use steam (thus limiting the temperature to 100 °C (212 °F) ) when ether must be heated or distilled. The diffusion of diethyl ether in air is 0.918·10−5 m2/s (298K, 101.325 kPa).[citation needed]
Ether is sensitive to light and air, tending to form explosive peroxides.[27] Ether peroxides are higher boiling than ether and are contact explosives when dry.[27] Commercial diethyl ether is typically supplied with trace amounts of the antioxidant butylated hydroxytoluene (BHT), which reduces the formation of peroxides. Storage over sodium hydroxide precipitates the intermediate ether hydroperoxides. Water and peroxides can be removed by either distillation from sodium and benzophenone, or by passing through a column of activated alumina.[28]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Template:PGCH
- ↑ Carl L. Yaws, Chemical Properties Handbook, McGraw-Hill, New York, 1999, page 567
- ↑ 3.0 3.1 "Ethyl Ether MSDS". J.T. Baker. Retrieved 2010-06-24.
- ↑ 4.0 4.1 4.2 4.3 Toski, Judith A; Bacon, Douglas R; Calverley, Rod K (2001). The history of Anesthesiology. In: Barash, Paul G; Cullen, Bruce F; Stoelting, Robert K. Clinical Anesthesia (4 ed.). Lippincott Williams & Wilkins. p. 3. ISBN 978-0-7817-2268-1.
- ↑ Hademenos, George J.; Murphree, Shaun; Zahler, Kathy; Warner, Jennifer M. (2008-11-12). McGraw-Hill's PCAT. McGraw-Hill. p. 39. ISBN 978-0-07-160045-3. Retrieved 2011-05-25.
- ↑ Dr. Frobenius (1729) "An account of a spiritus vini æthereus, together with several experiments tried therewith," Philosophical Transactions of the Royal Society (London), 36 : 283-289.
- ↑ 7.0 7.1 "Ethers, by Lawrence Karas and W. J. Piel". Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2004.
- ↑ "Extra Strength Starting Fluid: How it Works". Valvovine. Archived from the original on 2007-09-27. Retrieved 2007-09-05.
- ↑ The Merck Index, 10th Edition, Martha Windholz, editor, Merck & Co., Inc, Rahway, NJ, 1983, page 551
- ↑ H. H. Rowley, Wm. R. Reed (1951). "Solubility of Water in Diethyl Ether at 25 °". J. Am. Chem. Soc. 73 (6): 2960–2960. doi:10.1021/ja01150a531.
- ↑ Microsoft Word - RedListE2007.doc
- ↑ 12.0 12.1 Hill, John W. and Kolb, Doris K. Chemistry for changing times: 10th edition. Page 257. Pearson: Prentice Hall. Upper saddle river, New Jersey. 2004.
- ↑ Madden, M. Leslie (May 14, 2004). "Crawford Long (1815-1878)". New Georgia Encylcopedia. University of Georgia Press. Retrieved February 13, 2015.
- ↑ "Crawford W. Long". Doctors' Day. Southern Medical Association. Retrieved February 13, 2015.
- ↑ Grattan, N. Treatment of Uterine Haemorrhage. Provincial Medicine and Surgical Journal. Vol. 1, No. 6 (Nov. 7, 1840), p. 107.
- ↑ Calderone, F.A. (1935). J. Pharmacology Experimental Therapeutics. 55 (1): 24–39 Jpet.aspetjournals.org http://jpet.aspetjournals.org/cgi/reprint/55/1/24.pdf Jpet.aspetjournals.org Check
|url=value (help). Missing or empty|title=(help) - ↑ "Ether and its effects in Anesthesia".
- ↑ Morgan, G. Edward, Jr. et al. "Clinical Anesthesiology" 3rd Ed, p. 3. New York: Mc Graw-Hill. 2002
- ↑ The National druggist, Volume 47, June 1917, pp.220
- ↑ Zandberg, Adrian (2010). "Short Article "Villages … Reek of Ether Vapours": Ether Drinking in Silesia before 1939". Medical History. 54 (3): 387–396. doi:10.1017/s002572730000466x. PMC 2890321. PMID 20592886.
- ↑ Kaszycki, Nestor (2006-08-30). "Łemkowska Watra w Żdyni 2006 – pilnowanie ognia pamięci". Histmag.org – historia od podszewki (in Polish). Kraków, Poland: i-Press. Retrieved 2009-11-25.
Dawniej eteru używało się w lecznictwie do narkozy, ponieważ ma właściwości halucynogenne, a już kilka kropel inhalacji wystarczyło do silnego znieczulenia pacjenta. Jednak eter, jak każda ciecz, może teoretycznie być napojem. Łemkowie tę teorię praktykują. Mimo to, nazywanie skroplonego eteru – „kropki” – ich „napojem narodowym” byłoby przesadą. Chociaż stanowi to pewną część mitu „bycia Łemkiem”.
- ↑ 109. Aspergillus flavus mutant strain 241, blocked in aflatoxin biosynthesis, does not accumulate aflR transcript. Matthew P. Brown and Gary A. Payne, North Carolina State University, Raleigh, NC 27695 fgsc.net
- ↑ P. T. Normann, A. Ripel and J. Morland (1987). "Diethyl Ether Inhibits Ethanol Metabolism in Vivo by Interaction with Alcohol Dehydrogenase". Alcoholism: Clinical and Experimental Research. 11 (2): 163–166. doi:10.1111/j.1530-0277.1987.tb01282.x. PMID 3296835.
- ↑ Larry K. Keefer, William A. Garland, Neil F. Oldfield, James E. Swagzdis, and Bruce A. Mico (1985). "Inhibition of N-Nitrosodimethylamine Metabolism in Rats by Ether Anesthesia" (PDF). Cancer Research. 45 (11 Pt 1): 5457–60. PMID 4053020.
- ↑ Ethyl Ether, Chem. Economics Handbook. Menlo Park, Calif: SRI International. 1991.
- ↑ Cohen, Julius Berend (1920). A Class-book of Organic Chemistry, Volume 1. London: Macmillan and Co. p. 39.
- ↑ 27.0 27.1 27.2 27.3 http://www.chem.purdue.edu/chemsafety/safetyclass/SDS/GHS-Et2O.pdf
- ↑ W. L. F. Armarego and C. L. L. Chai (2003). Purification of laboratory chemicals. Boston: Butterworth-Heinemann. ISBN 978-0-7506-7571-0.
External links
| Wikimedia Commons has media related to [[commons:Lua error in Module:WikidataIB at line 428: attempt to index field 'wikibase' (a nil value).|Lua error in Module:WikidataIB at line 428: attempt to index field 'wikibase' (a nil value).]]. |
- Michael Faraday's announcement of ether as an anesthetic in 1818
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of diethyl ether, ddbonline.ddbst.de
- CDC - NIOSH Pocket Guide to Chemical Hazards
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages with citations lacking titles
- Pages with citations having bare URLs
- Pages with URL errors
- CS1 maint: Unrecognized language
- Pages with broken file links
- Chemical articles with unknown parameter in Chembox
- ECHA InfoCard ID from Wikidata
- Chembox having DSD data
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from August 2011
- Articles with invalid date parameter in template
- Commons category link from Wikidata
- Commons category link is on Wikidata using P373
- Ethers
- General anesthetics
- Dissociative drugs
- Ether solvents
- GABAA receptor positive allosteric modulators
- NMDA receptor antagonists
- Glycine receptor agonists