Diet and cancer
|
WikiDoc Resources for Diet and cancer |
|
Articles |
|---|
|
Most recent articles on Diet and cancer Most cited articles on Diet and cancer |
|
Media |
|
Powerpoint slides on Diet and cancer |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Diet and cancer at Clinical Trials.gov Trial results on Diet and cancer Clinical Trials on Diet and cancer at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Diet and cancer NICE Guidance on Diet and cancer
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Diet and cancer Discussion groups on Diet and cancer Patient Handouts on Diet and cancer Directions to Hospitals Treating Diet and cancer Risk calculators and risk factors for Diet and cancer
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Diet and cancer |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Dietary patterns, foods, nutrients, and other dietary constituents are closely associated with the risk for several types of cancer. And while it is not yet possible to provide quantitative estimates of the overall risks, it has been estimated that 35 percent of cancer deaths may be related to dietary factors[1].
Reducing-risk factors
Increasing evidence suggests that diets high in fiber-containing foods are associated with a reduced risk for cancer, especially cancer of the colon[2].
A few studies have also shown a reduced risk for cancers of the breast, rectum, oral cavity, pharynx, stomach, and other sites with diets rich in fruits, vegetables and grain products [3]. Numerous studies have found evidence that carotenoids reduce the risk of some cancers. The evidence is particularly strong for lung cancer[4], even after taking smoking into account. Vitamin C is found in fruits, particularly citrus fruits and juices, and in green vegetables, as well as in some fortified foods. Of a group of epidemiologic studies investigating the role of vitamin C, three-fourths found that vitamin C, or fruit rich in vitamin C, provides significant protection[5].
Contrary Evidence
More recent studies have cast doubt on the claim that dietary fiber reduces the risk of colon cancer.[6][7] Regarding prostate cancer, a major 2002 study concluded that "A low-fat, high-fiber diet heavy in fruits and vegetables has no impact on PSA levels in men over a four-year period, and does not affect the incidence of prostate cancer."[8]
The belief that dietary fiber prevents these cancers was based on epidemiological evidence showing a very low incidence in the developing world. Dr. Denis Burkitt was the main proponent of this theory, and his 1979 best-selling book, "Don't Forget Fibre in Your Diet," was translated into nine languages. Less well-known is his theory, mentioned in the book, that the squatting defecation posture used in the developing world is also a preventative factor, especially for colon diseases. Now that his fiber theory has failed numerous attempts to confirm it, his other theory is gaining more attention as a new direction for research.
Methionine metabolism
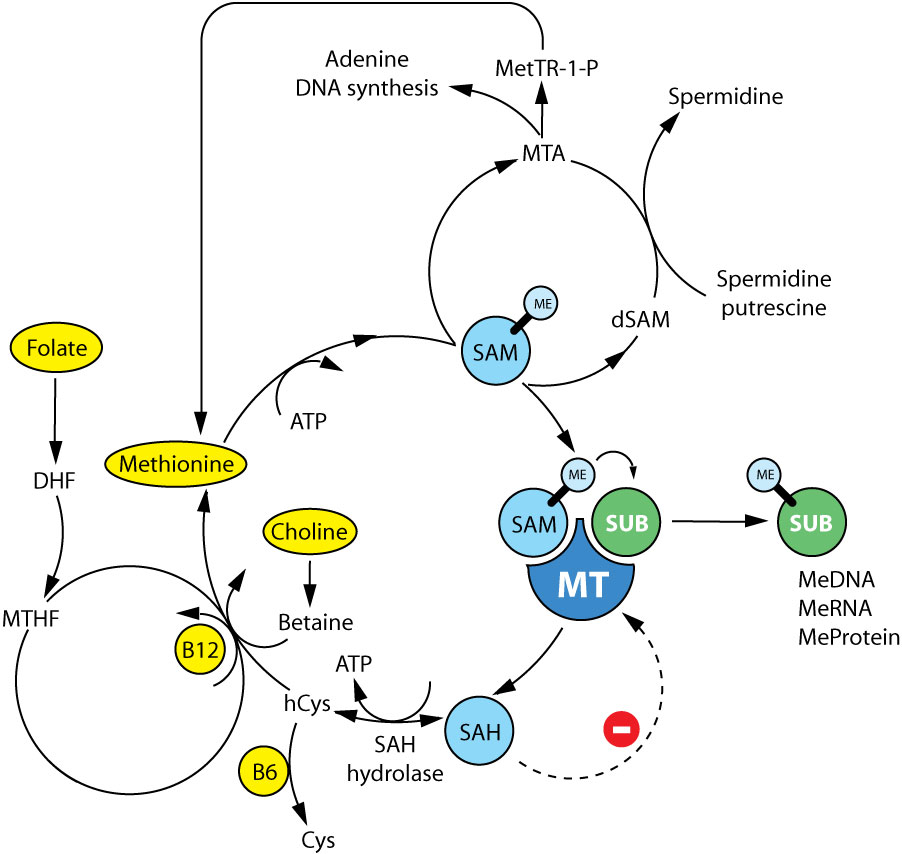
Although numerous cellular mechanisms are involved in food intake, many investigations over the past decades have pointed out defections in the methionine metabolic pathway as cause of cancerogenesis[9][10]. For instance, deficiencies of the main dietary sources of methyl donors, methionine and choline, lead to the formation of liver cancer in rodents[11][12]. Methionine is an essential amino acid that must be provided by dietary intake of proteins (meat) or methyl donors (choline and betaine found in beef, eggs and some vegetables). Assimilated methionine is transformed in S-adenosyl methionine (SAM) which is a key metabolite for polyamine synthesis, e.g. spermidine, and cysteine formation (see the figure on the right). Methionine breakdown products are also recycled back into methionine by homocysteine remethylation and methylthioadenosine (MTA) conversion (see the figure on the right). Vitamins B6, B12, folic acid and choline are essential cofactors for these reactions. SAM is the substrate for methylation reactions catalyzed by DNA, RNA and protein methyltransferases.
The products of these reactions are methylated DNA, RNA or proteins and S-adenosylhomocysteine (SAH). SAH has a negative feedback on its own production as an inhibitor of methyltransferase enzymes. Therefore SAM:SAH ratio directly regulates cellular methylation, whereas levels of vitamins B6, B12, folic acid and choline regulates indirectly the methylation state via the methionine metabolism cycle[13][14]. A near ubiquitous feature of cancer is a maladaption of the methionine metabolic pathway in response to genetic or environmental conditions resulting in depletion of SAM and/or SAM-dependent methylation. Whether it is deficiency in enzymes such as methylthioadenosine phosphorylase, methionine-dependency of cancer cells, high levels of polyamine synthesis in cancer, or induction of cancer through a diet deprived of extrinsic methyl donors or enhanced in methylation inhibitors, tumor formation is strongly correlated with a decrease in levels of SAM in mice, rats and humans[15][16]. Many indirect and thinly circumstantial theories have been put forth related to methylation status of DNA or attacks upon the capacity for DNA mutation and repair. The discovery that methyltransferases whose activity would be directly influenced by SAM levels also act as tumor suppressors potentially provides a more direct bridge. This has important ramifications for chemoprevention strategies as well as chemotherapy[17][18][19][20].
Arginine methyltransferase
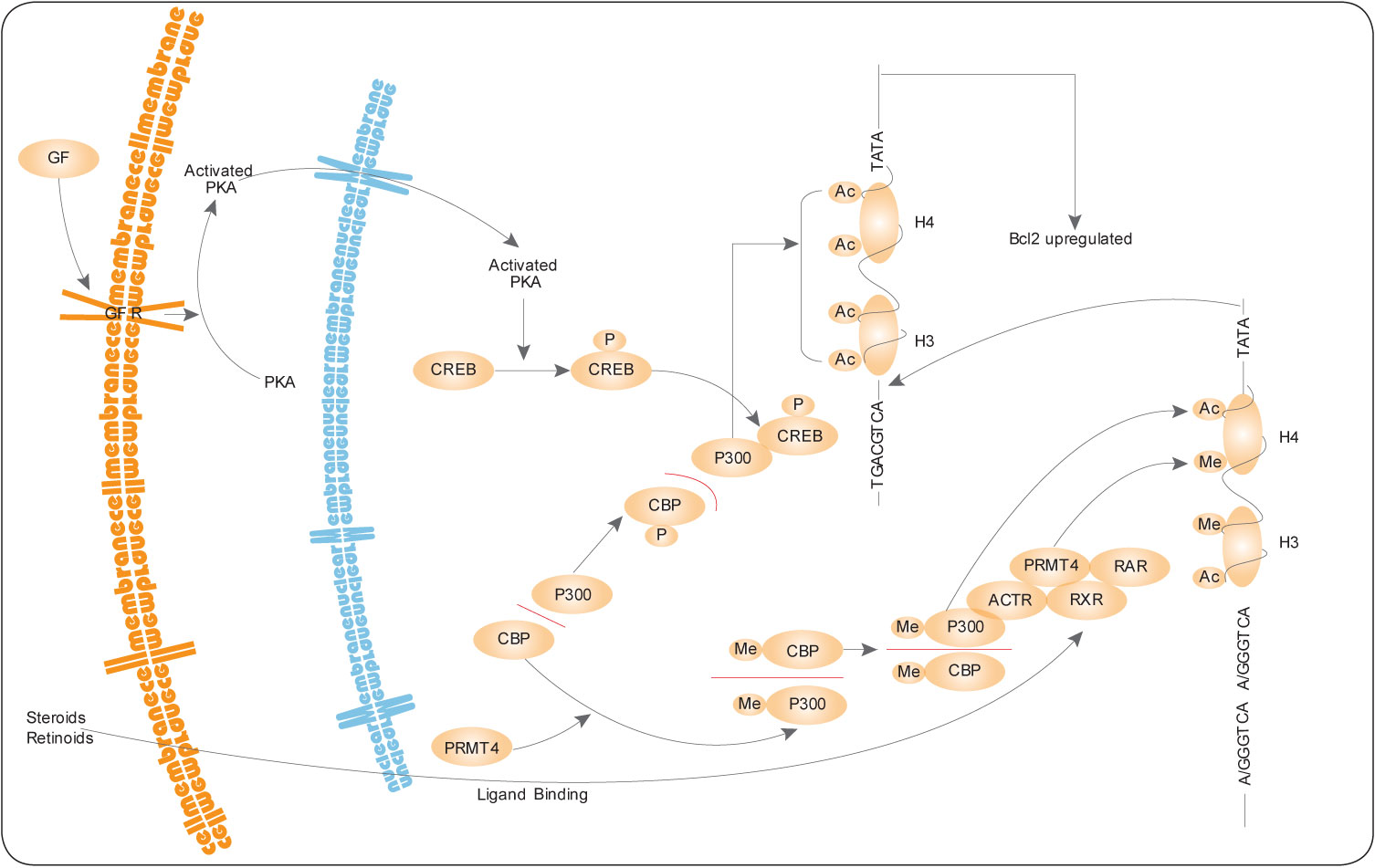
Protein arginine N-methyltransferase-4 (PRMT4) methylation of arginine residues within proteins plays a critical key role in transcriptional regulation (see the PRMT4 pathway on the left). PRMT4 binds to the classes of transcriptional activators known as p160 and CBP/p300[21]. The modified forms of these proteins are involved in stimulation of gene expression via steroid hormone receptors. Significantly, PRMT4 methylates core histones H3 and H4, which are also targets of the histone acetylase activity of CBP/p300 coactivators. PRMT4 recruitment chromatin by binding to coactivators increases histone methylation and enhances the accessibility of promoter regions for transcription. Methylation of the transcriptional coactivator CBP by PRMT4 inhibits binding to CREB and thereby partitions the limited cellular pool of CBP for steroid hormone receptor interaction.
References
- ↑ Doll R and Peto R: The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst (1981) 66:1191-1308
- ↑ Trock B, Lanza E and Greenwald P: Dietary fiber, vegetables and colon cancer: Critical review and meta-analysis of the epidemiologic evidence. J Natl Cancer Inst (1990) 82:650-661
- ↑ Lanza E, Shankar S and Trock B: Dietary fiber. In Macronutrients: Investigating their role in cancer. (1992) (Micozzi MS, Moon TE, eds). New York: Marcell Dekker, Inc., 293-319
- ↑ Ziegler RG: A review of epidemiologic evidence that carotenoids reduce the risk of cancer. (1989)J Nutr 119:116-122
- ↑ Block G: Vitamin C and cancer prevention: The epidemiologic evidence. Am J Clin Nutr (1991) 53:270S-282S
- ↑ The Boston Globe, "Doubts cast on fiber's effect on cancer" By Liz Kowalczyk, December 14,2005
- ↑ Associated Press, "Study: Fiber Doesn't Prevent Cancer" October 13, 2000
- ↑ Science Blog, August 2002,From American Society of Clinical Oncology
- ↑ Mikol, Y.B., Hoover, K.L., Creasia, D., Poirier, L.A. 1983. Carcinogenesis. 4: 1619-29
- ↑ Ghoshal, A.K., Farber, E. 1984. Carcinogenesis. 5: 1367-70
- ↑ Newmark, H.L., Yang, K., Lipkin, M., Kopelovich, L., Liu, Y., Fan, K., Shinozaki, H. 2001 Carcinogenesis. 11: 1871-5
- ↑ Henning, S.M., Swendseid, M.E., Coulson, W.F. 1997. J. Nutr. 127: 30-6
- ↑ Caudill, M.A., Wang, J.C., Melnyk, S., Pogribny, I.P., Jernigan, S., Collins, M.D., Santos-Guzman, J., Swendseid, M.E., Cogger, E.A., James, S.J. 2001. J. Nutr. 131(11): 2811-8
- ↑ Poirier, L.A., Wise, C.K., Delongchamp, R.R., Sinha, R. 2001. Cancer Epidemiol. Biomarkers Prev. 10: 649-55
- ↑ Prinz-Langenohl, R., Fohr, I., Pietrzik, K. 2001. Eur. J. Nut. 40: 98-105
- ↑ Van de Veyver, I.B. 2002. Annu. Rev. Nutr. 22: 2555-82
- ↑ McBride, A.E., Silver, P.A. 2001. Cell. 106: 5-8
- ↑ Huang, S. 2002. Nat. Rev. Cancer. 2: 469-76
- ↑ Rea, S., Eisenbaber, F., O’Carroll, D., Strahl, B.D., Sun, Z.W., Schmid, M. Opravil, S., Mechtler, K. Ponting, C.P., Allis, C.D., Jenuwein, T. 2000. Nature. 406: 593-9
- ↑ Steele-Perkins, G., Fang, W., Yang, X.H., Van Gele, M., Carling, T., Gu, J., Buyse, I.M., Fletcher, J.A., Liu, J., Bronson, R., Chadwick, R.B., de la Chapelle, A., Zhang, X., Speleman, F., Huang, S. Genes Dev. 2001. 15: 2250-62
- ↑ Zika E, et al., Interplay among coactivator-associated arginine methyltransferase 1, CBP, and CIITA in IFN-gamma-inducible MHC-II gene expression. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16321-6
See also
- DNA methyltransferase
- List of oncology-related terms
- Oncology
- American Cancer Society
- American Association for Cancer Research
- National Comprehensive Cancer Network
Template:Nutritional pathology Template:Tumors Template:WH Template:WS