Deferasirox
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RENAL FAILURE, HEPATIC FAILURE, AND GASTROINTESTINAL HEMORRHAGE
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Deferasirox is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Deferasirox in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Deferasirox in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Deferasirox in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Deferasirox in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Deferasirox in pediatric patients.
Contraindications
- Condition1
Warnings
|
WARNING: RENAL FAILURE, HEPATIC FAILURE, AND GASTROINTESTINAL HEMORRHAGE
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Description
Precautions
- Description
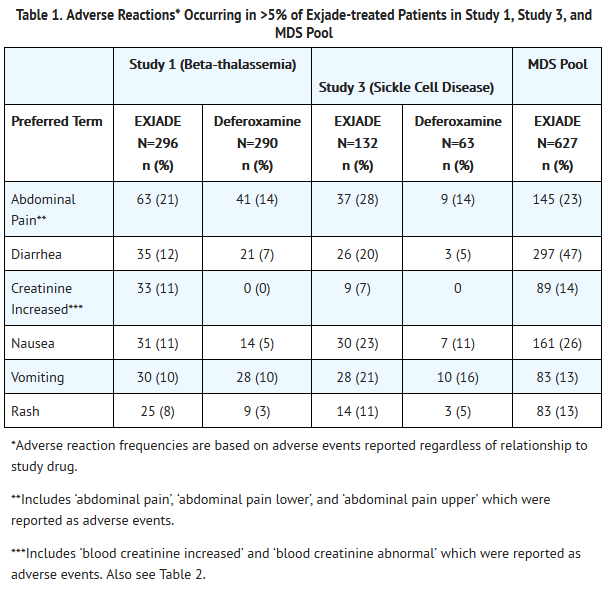
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Deferasirox in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Deferasirox in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Deferasirox in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Deferasirox during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Deferasirox with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Deferasirox with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Deferasirox with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Deferasirox with respect to specific gender populations.
Race
There is no FDA guidance on the use of Deferasirox with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Deferasirox in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Deferasirox in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Deferasirox in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Deferasirox in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Deferasirox in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Deferasirox in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Deferasirox in the drug label.
Pharmacology
There is limited information regarding Deferasirox Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Deferasirox in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Deferasirox in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Deferasirox in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Deferasirox in the drug label.
How Supplied
Storage
There is limited information regarding Deferasirox Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Deferasirox |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Deferasirox |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Deferasirox in the drug label.
Precautions with Alcohol
- Alcohol-Deferasirox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Deferasirox |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Deferasirox |Label Name=Deferasirox11.png
}}
{{#subobject:
|Label Page=Deferasirox |Label Name=Deferasirox11.png
}}