Dabigatran: Difference between revisions
Kiran Singh (talk | contribs) |
|||
| (89 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{ {{PAGENAME}} }}<div style="width: 80%"> | ||
__NOTOC__ | |||
{{CMG}}; {{AE}} {{SS}} | |||
{{SB}} Pradaxa® | |||
==Disclaimer== | |||
| | '''''WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs. Please read our full disclaimer [[wikidoc:General_disclaimer|{{fontcolor|#FF0000|here}}]].''''' | ||
| | |||
| | ==<span style="color:#FF0000; background:#000000;">Black Box Warning</span>== | ||
{| style="border: 3px solid #696969;" | |||
| style="background: #000000; border: 0px; padding: 20px 20px; width: 800px;" | | |||
| | <center> | ||
| | <font color="#F8F8FF" style="font-weight: bold;">WARNING: (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA</font> | ||
| | </center> | ||
<center> | |||
<font color="#F8F8FF" size="1" style="font-style: italic;">See full prescribing information for complete boxed warning.</font> | |||
</center> | |||
<font color="#F8F8FF" style="font-weight: bold;"> | |||
(A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS | |||
Premature discontinuation of any oral anticoagulant, including PRADAXA, increases the risk of thrombotic events. If anticoagulation with PRADAXA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant. | |||
(B) SPINAL/EPIDURAL HEMATOMA | |||
Epidural or spinal hematomas may occur in patients treated with PRADAXA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: | |||
* use of indwelling epidural catheters | |||
* concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants | |||
* a history of traumatic or repeated epidural or spinal punctures | |||
* a history of spinal deformity or spinal surgery | |||
* optimal timing between the administration of PRADAXA and neuraxial procedures is not known. | |||
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. | |||
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated.</font> | |||
|} | |||
==Overview== | ==Overview== | ||
{{PAGENAME}} is a [[direct thrombin inhibitor]] that is FDA approved for the prophylaxis of [[stroke]] and systemic [[embolism]] in patients with non-valvular [[atrial fibrillation]]. There is a Black Box Warning for this drug as shown <span style="background:#000000;">'''[[{{PAGENAME}}#Black Box Warning|{{fontcolor|#FF0000|here}}]]'''</span>. Common adverse reactions include [[esophagitis]], [[gastritis]], [[gastroesophageal reflux disease]], [[gastrointestinal hemorrhage]], gastrointestinal ulcer, [[indigestion]],[[bleeding]]. | |||
==Adult Indications and Dosage== | |||
===FDA-Labeled Indications and Dosage (Adult)=== | |||
=====Atrial fibrillation===== | |||
* Dosing Information | |||
:* Recommended dose for patients creatinine clearance (CrCl) >30 mL/min: '''150 mg PO bid''' | |||
:* Recommended dose for patients (CrCl 15-30 mL/min): '''75 mg PO bid''' | |||
:* Recommended dose for patients CrCl <15 mL/min or on dialysis: '''Not provided by FDA''' | |||
===Off-Label Use and Dosage (Adult)=== | |||
====Guideline-Supported Use==== | |||
=====Postoperative deep vein thrombosis; Prophylaxis===== | |||
* Developed by: [[American College of Chest Physicians|American College of Chest Physicians(ACCP)]] | |||
* Class of Recommendation: [[ACCP guidelines classification scheme#Class II:Benefit ≥ Risk]] | |||
* Level of Evidence:[[ACCP guidelines classification scheme#Level of Evidence B:|Level B]] | |||
* Recommendation | |||
:* '''220 mg or 150 mg PO qd''' | |||
=====Atrial fibrillation - Thromboembolic disorder; Prophylaxis===== | |||
* Developed by: [[American College of Chest Physicians|American College of Chest Physicians(ACCP)]] | |||
* Class of Recommendation: [[ACCP guidelines classification scheme#Class II:Benefit ≥ Risk]] | |||
* Level of Evidence:[[ACCP guidelines classification scheme#Level of Evidence B:|Level B]] | |||
* Recommendation | |||
:* '''150 mg PO bid''' <ref name="pmid22315265">Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S et al. (2012) [http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&retmode=ref&cmd=prlinks&id=22315265 Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines.] ''Chest'' 141 (2 Suppl):e278S-325S. [http://dx.doi.org/10.1378/chest.11-2404 DOI:10.1378/chest.11-2404] PMID: [http://pubmed.gov/22315265 22315265]</ref> | |||
=====Postoperative deep vein thrombosis; Prophylaxis===== | |||
* Developed by: [[American College of Chest Physicians|American College of Chest Physicians(ACCP)]] | |||
* Class of Recommendation: [[ACCP guidelines classification scheme#Class II:Benefit ≥ Risk]] | |||
* Level of Evidence:[[ACCP guidelines classification scheme#Level of Evidence B:|Level B]] | |||
* Recommendation | |||
=====Venous thromboembolism===== | |||
* Developed by: [[American College of Chest Physicians|American College of Chest Physicians(ACCP)]] | |||
* Class of Recommendation: [[ACCP guidelines classification scheme#Class II:Benefit ≥ Risk]] | |||
* Level of Evidence:[[ACCP guidelines classification scheme#Level of Evidence B:|Level B]] | |||
==Pediatric Indications and Dosage== | |||
* Safety and effectiveness of PRADAXA in pediatric patients have not been established. | |||
==Contraindications== | |||
* Active pathological bleeding. | |||
* History of a serious [[hypersensitivity]] reaction to PRADAXA (e.g., [[anaphylactic reaction]] or [[anaphylactic shock]]) . | |||
* Mechanical [[prosthetic heart valve]]. | |||
== | ==Warnings== | ||
== | =====Increased Risk of Stroke with Discontinuation of PRADAXA===== | ||
* Discontinuing PRADAXA in absence of adequate alternative anticoagulation increases the risk of thrombotic events. If PRADAXA must be discontinued for a reason other than pathological bleeding, consider coverage with another anticoagulant. | |||
== | =====Risk of Bleeding===== | ||
* PRADAXA increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue PRADAXA in patients with active pathological bleeding. | |||
PRADAXA increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding | |||
* Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). PRADAXA’s anticoagulant activity and half-life are increased in patients with renal impairment. | |||
* A specific reversal agent for dabigatran is not available. [[Hemodialysis]] can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited. Activated prothrombin complex concentrates (aPCCs, e.g., FEIBA), or recombinant Factor VIIa, or concentrates of coagulation factors II, IX or X may be considered but their use has not been evaluated in clinical trials. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of dabigatran. Consider administration of platelet concentrates in cases where [[thrombocytopenia]] is present or long-acting antiplatelet drugs have been used. | |||
===Effect of P-gp Inducers and Inhibitors on Dabigatran Exposure=== | =====Thromboembolic and Bleeding Events in Patients with Prosthetic Heart Valves===== | ||
The concomitant use of PRADAXA with P-gp inducers (e.g. | |||
inhibitors. | * The safety and efficacy of PRADAXA in patients with bileaflet mechanical prosthetic heart valves was evaluated in the RE-ALIGN trial, in which patients with bileaflet mechanical [[prosthetic heart valves]] (recently implanted or implanted more than three months prior to enrollment) were randomized to dose adjusted [[warfarin]] or 150, 220, or 300 mg of PRADAXA twice a day. RE-ALIGN was terminated early due to the occurrence of significantly more thromboembolic events (valve [[thrombosis]], [[stroke]], [[transient ischemic attack]], and [[myocardial infarction]]) and an excess of major bleeding (predominantly post-operative pericardial effusions requiring intervention for hemodynamic compromise) in the PRADAXA treatment arm as compared to the [[warfarin]] treatment arm. These bleeding and thromboembolic events were seen both in patients who were initiated on PRADAXA post-operatively within three days of mechanical bileaflet valve implantation, as well as in patients whose valves had been implanted more than three months prior to enrollment. Therefore, the use of PRADAXA is contraindicated in patients with mechanical [[prosthetic valves]]. | ||
* The use of PRADAXA for the prophylaxis of thromboembolic events in patients with [[atrial fibrillation]] in the setting of other forms of valvular heart disease, including the presence of a bioprosthetic heart valve, has not been studied and is not recommended. | |||
=====Effect of P-gp Inducers and Inhibitors on Dabigatran Exposure===== | |||
* The concomitant use of PRADAXA with P-gp inducers (e.g. rifampin) reduces exposure to dabigatran and should generally be avoided. | |||
* P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone. | |||
* Consider reducing the dose of PRADAXA to 75 mg twice daily when [[dronedarone]] or systemic [[ketoconazole]] is coadministered with PRADAXA in patients with moderate renal impairment (CrCl 30-50 mL/min). Avoid use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min). | |||
==Adverse Reactions== | ==Adverse Reactions== | ||
=== | ===Clinical Trials Experience=== | ||
In the RE-LY study, drug hypersensitivity (including [[urticaria]], [[rash]], and [[pruritus]]), allergic [[edema]], [[anaphylactic reaction]], and anaphylactic shock were reported in <0.1% of patients receiving PRADAXA. | |||
* Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
* The RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study provided safety information on the use of two doses of PRADAXA and warfarin. The numbers of patients and their exposures are described in Table 1. Limited information is presented on the 110 mg dosing arm because this dose is not approved. | |||
{| | |||
|- | |||
|[[File:Dabigatran02.jpg|thumb|800px|left]] | |||
|- | |||
|} | |||
<u>Drug Discontinuation in RE-LY</u> | |||
* The rates of adverse reactions leading to treatment discontinuation were 21% for PRADAXA 150 mg and 16% for warfarin. The most frequent adverse reactions leading to discontinuation of PRADAXA were bleeding and gastrointestinal events (i.e., [[dyspepsia]], [[nausea]], upper [[abdominal pain]], [[gastrointestinal hemorrhage]], and [[diarrhea]]). | |||
<u>Bleeding</u> | |||
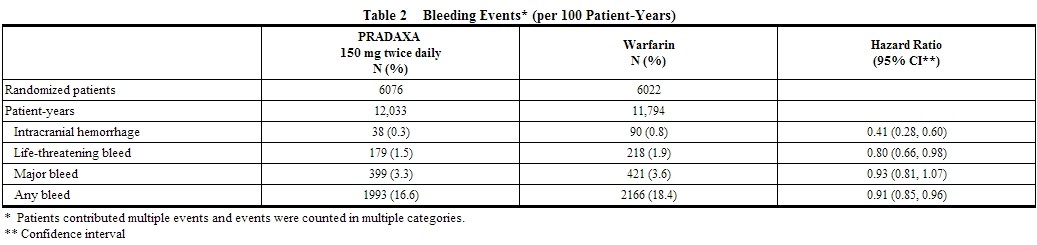
* Table 2 shows the number of patients experiencing serious bleeding during the treatment period in the RE-LY study, with the bleeding rate per 800 patient-years (%). Major bleeds fulfilled one or more of the following criteria: bleeding associated with a reduction in hemoglobin of at least 2 grams per deciliter or leading to a transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with [[compartment syndrome]], retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding). A life-threatening bleed met one or more of the following criteria: fatal, symptomatic intracranial bleed, reduction in hemoglobin of at least 5 grams per deciliter, transfusion of at least 4 units of blood, associated with hypotension requiring the use of intravenous inotropic agents, or necessitating surgical intervention. [[Intracranial hemorrhage]] included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds. | |||
{| | |||
|- | |||
|[[File:Dabigatran03.jpg|thumb|800px|left]] | |||
|- | |||
|} | |||
* The risk of major bleeds was similar with PRADAXA 150 mg and [[warfarin]] across major subgroups defined by baseline characteristics, with the exception of age, where there was a trend towards a higher incidence of major bleeding on PRADAXA (hazard ratio 1.2, 95% CI: 1.0 to 1.4) for patients ≥75 years of age. | |||
* There was a higher rate of major [[gastrointestinal bleeds]] in patients receiving PRADAXA 150 mg than in patients receiving [[warfarin]] (1.6% vs. 1.1%, respectively, with a hazard ratio vs. warfarin of 1.5, 95% CI, 1.2 to 1.9), and a higher rate of any [[gastrointestinal bleeds]] (6.1% vs. 4.0%, respectively). | |||
<u>Gastrointestinal Adverse Reactions</u> | |||
* Patients on PRADAXA 150 mg had an increased incidence of gastrointestinal adverse reactions (35% vs. 24% on [[warfarin]]). These were commonly [[dyspepsia]] (including [[abdominal pain]] upper, [[abdominal pain]], abdominal discomfort, and epigastric discomfort) and [[gastritis]]-like symptoms (including [[GERD]], [[esophagitis]], erosive [[gastritis]], [[gastric hemorrhage]], hemorrhagic [[gastritis]], hemorrhagic erosive [[gastritis]], and [[gastrointestinal ulcer]]). | |||
<u>Hypersensitivity Reactions</u> | |||
* In the RE-LY study, drug [[hypersensitivity]] (including [[urticaria]], [[rash]], and [[pruritus]]), allergic [[edema]], [[anaphylactic reaction]], and [[anaphylactic shock]] were reported in <0.1% of patients receiving PRADAXA. | |||
===Postmarketing=== | |||
* The following adverse reactions have been identified during post approval use of PRADAXA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during post approval use of PRADAXA: [[angioedema]], [[thrombocytopenia]], [[esophageal ulcer]]. | |||
==Drug Interactions== | |||
* The concomitant use of PRADAXA with [P-gp inducers] (e.g., [[rifampin]]) reduces exposure to dabigatran and should generally be avoided. | |||
* P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone. | |||
* In patients with moderate renal impairment (CrCl 30-50 mL/min), consider reducing the dose of PRADAXA to 75 mg twice daily when administered concomitantly with the P-gp inhibitor dronedarone or systemic ketoconazole. The use of P-gp inhibitors (verapamil, amiodarone, quinidine, and clarithromycin) does not require a dose adjustment of PRADAXA. These results should not be extrapolated to other P-gp inhibitors. | |||
* The concomitant use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min) should be avoided. | |||
* There are no adequate and well-controlled studies in pregnant women. | |||
* Dabigatran has been shown to decrease the number of implantations when male and female rats were treated at a dosage of 70 mg/kg (about 2.6 to 3.0 times the human exposure at maximum recommended human dose [MRHD] of 300 mg/day based on area under the curve [AUC] comparisons) prior to mating and up to implantation (gestation Day 6). Treatment of pregnant rats after implantation with dabigatran at the same dose increased the number of dead offspring and caused excess vaginal/uterine bleeding close to parturition. Although dabigatran increased the incidence of delayed or irregular ossification of fetal skull bones and vertebrae in the rat, it did not induce major malformations in rats or rabbits. | |||
==Use in Specific Populations== | ==Use in Specific Populations== | ||
===Labor and Delivery=== | ====Pregnancy==== | ||
Safety and effectiveness of PRADAXA during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using PRADAXA in this setting. Death of offspring and mother rats during labor in association with uterine bleeding occurred during treatment of pregnant rats from implantation (gestation Day 7) to | |||
weaning (lactation Day 21) with dabigatran at a dose of 70 mg/kg (about 2.6 times the human exposure at MRHD of 300 mg/day based on AUC comparisons). | ====='''[[Pregnancy category#United States|Pregnancy Category (FDA)]]: C'''===== | ||
====='''[[Pregnancy category#Australia|Pregnancy Category (AUS)]]: {{PAGENAME}} is not included in Australian Drug Evaluation Committee (ADEC) Pregnancy Categories.'''===== | |||
====Labor and Delivery==== | |||
*Safety and effectiveness of PRADAXA during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using PRADAXA in this setting. | |||
*Death of offspring and mother rats during labor in association with uterine bleeding occurred during treatment of pregnant rats from implantation (gestation Day 7) to weaning (lactation Day 21) with dabigatran at a dose of 70 mg/kg (about 2.6 times the human exposure at MRHD of 300 mg/day based on AUC comparisons). | |||
====Nursing Mothers==== | |||
*It is not known whether dabigatran is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PRADAXA is administered to a nursing woman. | |||
====Pediatric Use==== | |||
*Safety and effectiveness of PRADAXA in pediatric patients have not been established. | |||
====Geriatric Use==== | |||
* Of the total number of patients in the RE-LY study, 82% were 65 and over, while 40% were 75 and over. The risk of stroke and bleeding increases with age, but the risk-benefit profile is favorable in all age groups. | |||
* Use In Renal Impair=No dose adjustment of PRADAXA is recommended in patients with mild or moderate renal impairment. Reduce the dose of PRADAXA in patients with severe renal impairment (CrCl 15-30 mL/min). Dosing recommendations for patients with CrCl <15 mL/min or on dialysis cannot be provided. | |||
* Adjust dose appropriately in patients with renal impairment receiving concomitant P-gp inhibitors. | |||
====Gender==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Gender''. | |||
====Race==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Race''. | |||
====Renal Impairment==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Renal Impairment''. | |||
====Hepatic Impairment==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Hepatic Impairment''. | |||
====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
*Dabigatran was not carcinogenic when administered by oral gavage to mice and rats for up to 2 years. The highest doses tested (200 mg/kg/day) in mice and rats were approximately 3.6 and 6 times, respectively, the human exposure at MRHD of 300 mg/day based on AUC comparisons. | |||
*Dabigatran was not mutagenic in in vitro tests, including bacterial reversion tests, mouse lymphoma assay and chromosomal aberration assay in human lymphocytes, and thein vivo micronucleus assay in rats. | |||
*In the rat fertility study with oral gavage doses of 15, 70, and 200 mg/kg, males were treated for 29 days prior to mating, during mating up to scheduled termination, and females were treated 15 days prior to mating through gestation Day 6. No adverse effects on male or female fertility were observed at 200 mg/kg or 9 to 12 times the human exposure at MRHD of 300 mg/day based on AUC comparisons. However, the number of implantations decreased in females receiving 70 mg/kg, or 3 times the human exposure at MRHD based on AUC comparisons. | |||
====Immunocompromised Patients==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Immunocompromised Patients''. | |||
====Miscellaneous==== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Miscellaneous''. | |||
==Administration and Monitoring== | |||
====Administration==== | |||
* Oral | |||
===Instructions to Patients=== | |||
* Instruct patients to swallow the capsules whole. PRADAXA should be taken with a full glass of water. Breaking, chewing, or emptying the contents of the capsule can result in increased exposure. | |||
* If a dose of PRADAXA is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. The dose of PRADAXA should not be doubled to make up for a missed dose. | |||
===Converting from or to Warfarin=== | |||
* When converting patients from warfarin therapy to PRADAXA, discontinue warfarin and start PRADAXA when the INR is below 2.0. | |||
* When converting from PRADAXA to warfarin, adjust the starting time of warfarin based on creatinine clearance as follows: | |||
:*For CrCl ≥50 mL/min, start warfarin 3 days before discontinuing PRADAXA. | |||
:*For CrCl 30-50 mL/min, start warfarin 2 days before discontinuing PRADAXA. | |||
:*For CrCl 15-30 mL/min, start warfarin 1 day before discontinuing PRADAXA. | |||
:*For CrCl <15 mL/min, no recommendations can be made. | |||
* Because PRADAXA can increase INR, the INR will better reflect warfarin’s effect only after PRADAXA has been stopped for at least 2 days. | |||
===Converting from or to Parenteral Anticoagulants=== | |||
* For patients currently receiving a parenteral anticoagulant, start PRADAXA 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (e.g., intravenous unfractionated heparin). | |||
* For patients currently taking PRADAXA, wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of PRADAXA before initiating treatment with a parenteral anticoagulant. | |||
===Surgery and Interventions=== | |||
* If possible, discontinue PRADAXA 1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required. | |||
* If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention. | |||
* Monitoring=INR is relatively insensitive to the exposure to dabigatran and cannot be interpreted the same way as used for warfarin monitoring. | |||
= | * IV Compat=FDA Package Insert for Metoprolol tartrate contains no information regarding Black Box Warning. | ||
= | * Overdose=Accidental overdose may lead to hemorrhagic complications. There is no reversal agent for dabigatran. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with PRADAXA, and investigate the source of bleeding. Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy | ||
=== | ==IV Compatibility== | ||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''IV Compatibility''. | |||
==Overdosage== | ==Overdosage== | ||
== | * Accidental overdose may lead to hemorrhagic complications. There is no reversal agent for dabigatran. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with PRADAXA, and investigate the source of bleeding. Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy. | ||
==Pharmacology== | |||
===Mechanism of Action=== | ===Mechanism of Action=== | ||
Dabigatran and its acyl glucuronides are competitive, | |||
are inhibited by the active moieties. | * Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors. Because thrombin (serine protease) enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus. Both free and clot-bound thrombin, and thrombin-induced platelet aggregation are inhibited by the active moieties. | ||
===Structure=== | |||
{{drugbox2 | |||
| verifiedrevid = 407639691 | |||
| drug_name = Dabigatran etexilate | |||
| IUPAC_name = Ethyl 3-{[(2-{[(4-{''N''{{'}}-hexyloxycarbonyl carbamimidoyl}phenyl)amino]methyl}-1- | |||
methyl-1''H''-benzimidazol-5-yl)carbonyl] (pyridin-2-yl-amino)propanoate | |||
| image = Dabigatran etexilate structure.png | |||
| width = 135 | |||
| image2 = Dabigatran etexilate ball-and-stick.png | |||
| width2 = 250 | |||
| tradename = Pradaxa,Prazaxa | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4948999 | |||
| InChI = 1/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44) | |||
| smiles = O=C(OCC)CCN(c1ncccc1)C(=O)c4ccc2c(nc(n2C)CNc3ccc(C(=N\C(=O)OCCCCCC)\N)cc3)c4 | |||
| InChIKey = KSGXQBZTULBEEQ-UHFFFAOYAL | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 539697 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = KSGXQBZTULBEEQ-UHFFFAOYSA-N | |||
| CAS_number = 211915-06-9 | |||
| ATC_prefix = B01 | |||
| ATC_suffix = AE07 | |||
| ATC_supplemental= | |||
| PubChem = 6445226 | |||
| DrugBank = DB06695 | |||
| chemical_formula = | |||
| C=34 | H=41 | N=7 | O=5 | |||
| molecular_weight = 627.734 g/mol | |||
| specific_rotation = | |||
| sec_combustion = | |||
| bioavailability = 3–7%<ref name=prescribing_info>{{cite web|url=http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf|title= Pradaxa Full Prescribing Information|publisher=[[Boehringer Ingelheim]]|accessdate=October 2010}}</ref> | |||
| protein_bound = 35%<ref name=prescribing_info /> | |||
| metabolism = | |||
| elimination_half-life = 12–17 hours<ref name=prescribing_info /> | |||
| excretion = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = C | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | |||
| legal_CA = Schedule VI | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| dependency_liability = | |||
| routes_of_administration = oral | |||
| licence_EU = Pradaxa | |||
| licence_US = Dabigatran | |||
| MedlinePlus = a610024 | |||
}} | |||
===Pharmacodynamics=== | ===Pharmacodynamics=== | ||
===Cardiac Electrophysiology=== | * At recommended therapeutic doses, dabigatran etexilate prolongs the coagulation markers such as aPTT, ECT, and TT. INR is relatively insensitive to the exposure to dabigatran and cannot be interpreted the same way as used for [[warfarin]] monitoring. | ||
No prolongation of the QTc interval was observed with dabigatran etexilate at doses up to 600 mg. | |||
* The aPTT test provides an approximation of PRADAXA’s anticoagulant effect. The average time course for effects on aPTT, following approved dosing regimens in patients with various degrees of renal impairment is shown in Figure 1. The curves represent mean levels without confidence intervals; variations should be expected when measuring aPTT. While advice cannot be provided on the level of recovery of aPTT needed in any particular clinical setting, the curves can be used to estimate the time to get to a particular level of recovery, even when the time since the last dose of PRADAXA is not precisely known. In the RE-LY trial, the median (10th to 90th percentile) trough aPTT in patients receiving the 150 mg dose was 52 (40 to 76) seconds. | |||
*Simulations based on PK data from a study in subjects with renal impairment and PK/aPTT relationships derived from the RE-LY study; aPTT prolongation in RE-LY was measured centrally in citrate plasma using PTT Reagent Roche Diagnostics GmbH, Mannheim, Germany. There may be quantitative differences between various established methods for aPTT assessment. | |||
* The degree of anticoagulant activity can also be assessed by the ecarin clotting time (ECT). This test is a more specific measure of the effect of dabigatran than activated partial thromboplastin time (aPTT). In the RE-LY trial, the median (10th to 90th percentile) trough ECT in patients receiving the 150 mg dose was 63 (44 to 103) seconds. | |||
====Cardiac Electrophysiology==== | |||
* No prolongation of the QTc interval was observed with dabigatran etexilate at doses up to 600 mg. | |||
===Pharmacokinetics=== | ===Pharmacokinetics=== | ||
* Dabigatran etexilate mesylate is absorbed as the dabigatran etexilate ester. The ester is then hydrolyzed, forming dabigatran, the active moiety. Dabigatran is metabolized to four different acyl glucuronides and both the glucuronides and dabigatran have similar pharmacological activity. Pharmacokinetics described here refer to the sum of dabigatran and its glucuronides. Dabigatran displays dose-proportional pharmacokinetics in healthy subjects and patients in the range of doses from 10 to 400 mg. | |||
====Distribution==== | ====Distribution==== | ||
Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran’s accumulation factor is approximately two. | |||
* Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran’s accumulation factor is approximately two. | |||
====Elimination==== | ====Elimination==== | ||
Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran is 80% of total clearance after intravenous administration. After oral administration of radiolabeled dabigatran, 7% of radioactivity is recovered in urine and 86% in feces. The half-life of dabigatran in healthy subjects is 12 to 17 hours. | |||
* Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran is 80% of total clearance after intravenous administration. After oral administration of radiolabeled dabigatran, 7% of radioactivity is recovered in urine and 86% in feces. The half-life of dabigatran in healthy subjects is 12 to 17 hours. | |||
====Metabolism==== | ====Metabolism==== | ||
After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma. | |||
* After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma. | |||
====Renal Impairment==== | ====Renal Impairment==== | ||
{| | * An open, parallel-group single-center study compared dabigatran pharmacokinetics in healthy subjects and patients with mild to moderate renal impairment receiving a single dose of PRADAXA 150 mg. Exposure to dabigatran increases with severity of renal function impairment (Table 3). Similar findings were observed in the RE-LY trial. | ||
{| | |||
|- | |- | ||
| | |[[File:Dabigatran06.jpg|thumb|600px|left]] | ||
|- | |- | ||
|} | |} | ||
====Hepatic Impairment==== | ====Hepatic Impairment==== | ||
Administration of PRADAXA in patients with moderate hepatic impairment (Child-Pugh B) showed a large inter-subject variability, but no evidence of a consistent change in exposure or pharmacodynamics. | |||
* Administration of PRADAXA in patients with moderate hepatic impairment (Child-Pugh B) showed a large inter-subject variability, but no evidence of a consistent change in exposure or pharmacodynamics. | |||
====Drug Interactions==== | ====Drug Interactions==== | ||
''' | * Impact of Other Drugs on Dabigatran | ||
====P-gp Inducers==== | |||
* Rifampin: Rifampin 600 mg once daily for 7 days followed by a single dose of dabigatran decreased its AUC and Cmax by 66% and 67%, respectively. By Day 7 after cessation of rifampin treatment, dabigatran exposure was close to normal. | |||
====P-gp Inhibitors==== | |||
* In studies with the P-gp inhibitors ketoconazole, amiodarone, verapamil, and quinidine, the time to peak, terminal half-life, and mean residence time of dabigatran were not affected. Any observed changes in Cmax and AUC are described below. | |||
'''Dronedarone''': Simultaneous administration of dabigatran etexilate and dronedarone (administered once or twice daily) increases exposure to dabigatran by 70 to 140% compared to dabigatran alone. The increase in exposure is only 30 to 60% higher compared to dabigatran alone when dronedarone is administered 2 hours after dabigatran etexilate. | |||
'''Ketoconazole''': Systemic ketoconazole increased dabigatran AUC and Cmax values by 138% and 135%, respectively, after a single dose of 400 mg, and 153%, and 149%, respectively, after multiple daily doses of 400 mg. | |||
'''Verapamil''': When dabigatran etexilate was coadministered with oral verapamil, the Cmax and AUC of dabigatran were increased. The extent of increase depends on the formulation of verapamil and timing of administration. If verapamil is present in the gut when dabigatran is taken, it will increase exposure to dabigatran with the greatest increase observed when a single dose of immediate-release verapamil is given one hour prior to dabigatran (AUC increased by a factor of 2.4). If verapamil is given 2 hours after dabigatran, the increase in AUC is negligible. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received verapamil. | |||
'''Amiodarone''': When dabigatran etexilate was coadministered with a single 600 mg oral dose of amiodarone, the dabigatran AUC and Cmax increased by 58% and 50%, respectively. The increase in exposure was mitigated by a 65% increase in the renal clearance of dabigatran in the presence of amiodarone. The increase in renal clearance may persist after amiodarone is discontinued because of amiodarone’s long half-life. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received amiodarone. | |||
'''Quinidine''': Quinidine was given as 200 mg dose every 2 hours up to a total dose of 1000 mg. Dabigatran etexilate was given over 3 consecutive days, the last evening dose on Day 3 with or without quinidine pre-dosing. Concomitant quinidine administration increased dabigatran’s AUC and Cmax by 53% and 56%, respectively. | |||
'''Clarithromycin''': Coadministered clarithromycin had no impact on the exposure to dabigatran. | |||
====Other Drugs==== | |||
'''Clopidogrel''': When dabigatran etexilate was given concomitantly with a loading dose of 300 mg or 600 mg clopidogrel, the dabigatran AUC and Cmax increased by approximately 30% and 40%, respectively. The concomitant administration of dabigatran etexilate and clopidogrel resulted in no further prolongation of capillary bleeding times compared to clopidogrel monotherapy. When comparing combined treatment and the respective mono-treatments, the coagulation measures for dabigatran’s effect (aPTT, ECT, and TT) remained unchanged, and inhibition of platelet aggregation (IPA), a measurement of clopidogrel’s effect, remained unchanged. | |||
'''Enoxaparin''': Enoxaparin 40 mg given subcutaneously for 3 days with the last dose given 24 hours before a single dose of PRADAXA had no impact on the exposure to dabigatran or the coagulation measures aPTT, ECT, or TT. | |||
'''Diclofenac, Ranitidine, and Digoxin''':None of these drugs alters exposure to dabigatran. | |||
In RE-LY, dabigatran plasma samples were also collected. The concomitant use of [[proton pump inhibitors]], [[H2 antagonists]], and [[digoxin]] did not appreciably change the trough concentration of dabigatran. | |||
'''Impact of Dabigatran on Other Drugs''' | |||
* In clinical studies exploring CYP3A4, CYP2C9, P-gp and other pathways, dabigatran did not meaningfully alter the pharmacokinetics of amiodarone, atorvastatin, clarithromycin, diclofenac, clopidogrel, digoxin, pantoprazole, or ranitidine. | |||
===Nonclinical Toxicology=== | |||
* FDA Package Insert for {{PAGENAME}} contains no information regarding ''Nonclinical Toxicology''. | |||
==Clinical Studies== | |||
* The clinical evidence for the efficacy of PRADAXA was derived from RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy), a multi-center, multi-national, randomized parallel group trial comparing two blinded doses of PRADAXA (110 mg twice daily and 150 mg twice daily) with open-label [[warfarin]] (dosed to target INR of 2 to 3) in patients with non-valvular, persistent, paroxysmal, or permanent [[atrial fibrillation]] and one or more of the following additional risk factors: | |||
*Previous stroke, transient ischemic attack (TIA), or systemic embolism | |||
*Left ventricular ejection fraction <40% | |||
*Symptomatic heart failure, ≥ New York Heart Association Class 2 | |||
*Age ≥75 years | |||
*Age ≥65 years and one of the following: diabetes mellitus, coronary artery disease (CAD), or hypertension | |||
* The primary objective of this study was to determine if PRADAXA was non-inferior to warfarin in reducing the occurrence of the composite endpoint, stroke (ischemic and hemorrhagic) and systemic embolism. The study was designed to ensure that PRADAXA preserved more than 50% of warfarin’s effect as established by previous randomized, placebo-controlled trials of warfarin in atrial fibrillation. Statistical superiority was also analyzed. | |||
* A total of 18,113 patients were randomized and followed for a median of 2 years. The patient’s mean age was 71.5 years and the mean CHADS2 score was 2.1. The patient population was 64% male, 70% Caucasian, 16% Asian, and 1% black. Twenty percent of patients had a history of a stroke or TIA and 50% were Vitamin K antagonist (VKA) naïve, defined as less than 2 months total lifetime exposure to a VKA. Thirty-two percent of the population had never been exposed to a VKA. Concomitant diseases of patients in this trial included hypertension 79%, diabetes 23%, and CAD 28%. At baseline, 40% of patients were on aspirin and 6% were on clopidogrel. For patients randomized to warfarin, the mean percentage of time in therapeutic range (INR 2 to 3) was 64%. | |||
* Relative to warfarin and to PRADAXA 110 mg twice daily, PRADAXA 150 mg twice daily significantly reduced the primary composite endpoint of stroke and systemic embolism (see Table 4 and Figure 2). | |||
{| | |||
|- | |||
|[[File:Dabigatran07.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran08.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
* The contributions of the components of the composite endpoint, including stroke by subtype, are shown in Table 5. The treatment effect was primarily a reduction in stroke. PRADAXA 150 mg twice daily was superior in reducing ischemic and hemorrhagic strokes relative to [[warfarin]]. | |||
{| | |||
|- | |||
|[[File:Dabigatran09.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
* In the RE-LY trial, the rate of all-cause mortality was lower on dabigatran 150 mg than on warfarin (3.6% per year versus 4.1% per year). The rate of vascular death was lower on dabigatran 150 mg compared to warfarin (2.3% per year versus 2.7% per year). Non-vascular death rates were similar in the treatment arms. | |||
* The efficacy of PRADAXA 150 mg twice daily was generally consistent across major subgroups (see Figure 3). | |||
{| | |||
|- | |||
|[[File:Dabigatran10.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
* In RE-LY, a higher rate of clinical myocardial infarction was reported in patients who received PRADAXA (0.7 per 100 patient-years for 150 mg dose) than in those who received warfarin (0.6). | |||
==How supplied== | |||
* PRADAXA 75 mg capsules have a cream-colored opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R75". The color of the imprinting is black. The capsules are supplied in the packages listed: | |||
*NDC 0597-0149-54 Unit of use bottle of 60 capsules | |||
*NDC 0597-0149-60 Blister package containing 60 capsules (10 x 6 capsule blister cards) | |||
* PRADAXA 150 mg capsules have a light blue opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R150". The color of the imprinting is black. The capsules are supplied in the packages listed: | |||
*NDC 0597-0135-54 Unit of use bottle of 60 capsules | |||
*NDC 0597-0135-60 Blister package containing 60 capsules (10 x 6 capsule blister cards) | |||
===Bottles=== | |||
* Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Once opened, the product must be used within 4 months. Keep the bottle tightly closed. Store in the original package to protect from moisture. | |||
===Blisters=== | |||
* Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Store in the original package to protect from moisture. | |||
==Images== | |||
===Drug Images=== | |||
{| | |||
| [[File:dabigatran 75mg01.jpg|thumb|300px|<p style="text-align: left;"> | |||
'''Drug Name''': Pradaxa 75 MG Oral Capsule <BR> | |||
'''Ingredient(s)''': Dabigatran etexilate <BR> | |||
'''Imprint''': R75 <BR> | |||
'''Color(s)''': White <BR> | |||
'''Shape''': Capsule <BR> | |||
'''Size (mm)''': 18.00 <BR> | |||
'''Score''': 1 <BR> | |||
'''Drug Label Author''': Boehringer Ingelheim Pharmaceuticals Inc. ]]</p> | |||
|} | |||
{| | |||
| [[File:dabigatran 75mg02.jpg|thumb|300px|<p style="text-align: left;"> | |||
'''Drug Name''': Pradaxa 75 MG Oral Capsule <BR> | |||
'''Ingredient(s)''': Dabigatran etexilate <BR> | |||
'''Imprint''': R75 <BR> | |||
'''Color(s)''': White <BR> | |||
'''Shape''': Capsule <BR> | |||
'''Size (mm)''': 18.00 <BR> | |||
'''Score''': 1 <BR> | |||
'''Drug Label Author''': Boehringer Ingelheim Pharmaceuticals Inc. ]]</p> | |||
|} | |||
{| | |||
| [[File:dabigatran 150mg01.jpg|thumb|300px|<p style="text-align: left;"> | |||
'''Drug Name''': Pradaxa 150 MG Oral Capsule <BR> | |||
'''Ingredient(s)''': Dabigatran etexilate <BR> | |||
'''Imprint''': R75 <BR> | |||
'''Color(s)''': White <BR> | |||
'''Shape''': Capsule <BR> | |||
'''Size (mm)''': 18.00 <BR> | |||
'''Score''': 1 <BR> | |||
'''Drug Label Author''': Boehringer Ingelheim Pharmaceuticals Inc. ]]</p> | |||
|} | |||
===Package and Label Display Panel=== | |||
{| | |||
|- | |||
|[[File:Dabigatran11.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran12.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran13.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran14.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran15.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran16.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran17.jpg|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran panel03.png|thumb|600px|left]] | |||
|- | |||
|} | |||
{| | |||
|- | |||
|[[File:Dabigatran panel01.png|thumb|600px|left]] | |||
|- | |||
|} | |||
[[ | {| | ||
|- | |||
|[[File:Dabigatran panel02.png|thumb|600px|left]] | |||
|- | |||
|} | |||
==Patient Information== | |||
===== | ===Patient Information from FDA=== | ||
* Advise the patient to read the FDA-approved patient labeling (Medication Guide). | |||
== | ===Instructions for Patients=== | ||
== | |||
*Tell patients to take PRADAXA exactly as prescribed. | |||
*Remind patients not to discontinue PRADAXA without talking to the health care provider who prescribed it. | |||
*Keep PRADAXA in the original bottle to protect from moisture. Do not put PRADAXA in pill boxes or pill organizers. | |||
*When more than one bottle is dispensed to the patient, instruct them to open only one bottle at a time. | |||
*Instruct patient to remove only one capsule from the opened bottle at the time of use. The bottle should be immediately and tightly closed. | |||
*Advise patients not to chew or break the capsules before swallowing them and not to open the capsules and take the pellets alone. | |||
*Advise patients that the capsule should be taken with a full glass of water. | |||
==== | ===Bleeding=== | ||
* Inform patients that they may bleed more easily, may bleed longer, and should call their health care provider for any signs or symptoms of bleeding. | |||
* Instruct patients to seek emergency care right away if they have any of the following, which may be a sign or symptom of serious bleeding: | |||
:*Unusual bruising (bruises that appear without known cause or that get bigger) | |||
:*Pink or brown urine | |||
:*Red or black, tarry stools | |||
:*Coughing up blood | |||
:*Vomiting blood, or vomit that looks like coffee grounds | |||
* Instruct patients to call their health care provider or to get prompt medical attention if they experience any signs or symptoms of bleeding: | |||
:*Pain, swelling or discomfort in a joint | |||
:*Headaches, dizziness, or weakness | |||
:*Reoccurring nose bleeds | |||
:*Unusual bleeding from gums | |||
:*Bleeding from a cut that takes a long time to stop | |||
:*Menstrual bleeding or vaginal bleeding that is heavier than normal | |||
== | ===Gastrointestinal Adverse Reactions=== | ||
== | |||
* Instruct patients to call their health care provider if they experience any signs or symptoms of dyspepsia or gastritis: | |||
:*Dyspepsia (upset stomach), burning, or nausea | |||
:*Abdominal pain or discomfort | |||
:*Epigastric discomfort, GERD (gastric indigestion) | |||
=== | ===Invasive or Surgical Procedures=== | ||
* Instruct patients to inform their health care provider that they are taking PRADAXA before any invasive procedure (including dental procedures) is scheduled. | |||
=== | ===Concomitant Medications=== | ||
* Ask patients to list all prescription medications, over-the-counter medications, or dietary supplements they are taking or plan to take so their health care provider knows about other treatments that may affect bleeding risk (e.g., aspirin or NSAIDs) or dabigatran exposure (e.g., dronedarone or systemic ketoconazole). | |||
=== | ===Prosthetic Heart Valves=== | ||
* Instruct patients to inform their health care provider if they will have or have had surgery to place a prosthetic heart valve. | |||
===Patient Information from NLM=== | |||
* For patient information about dabigatran from NLM, click [[Dabigatran (patient information)|here]]. | |||
==Precautions with Alcohol== | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
== | ==Brand Names== | ||
* Pradaxa® | |||
== | ==Look-Alike Drug Names== | ||
[ | ==[http://www.fda.gov/drugs/drugsafety/drugshortages/ucm050792.htm Drug Shortage Status]== | ||
==[ | ==[http://www.goodrx.com/{{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}}/price Price]== | ||
==References== | ==References== | ||
{{reflist}} | |||
</div> | |||
[[Category: | [[Category:Drug]] | ||
[[Category: | [[Category:Cardiovascular Drugs]] | ||
[[Category: | [[Category:Direct thrombin inhibitors]] | ||
Latest revision as of 14:17, 23 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Synonyms / Brand Names: Pradaxa®
Disclaimer
WikiDoc Drug Project is a constellation of drug information for healthcare providers and patients vigorously vetted on the basis of FDA package insert, MedlinePlus, Practice Guidelines, Scientific Statements, and scholarly medical literature. The information provided is not a medical advice or treatment. WikiDoc does not promote any medication or off-label use of drugs. Please read our full disclaimer here.
Black Box Warning
|
WARNING: (A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS, (B) SPINAL/EPIDURAL HEMATOMA See full prescribing information for complete boxed warning.
(A) PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS Premature discontinuation of any oral anticoagulant, including PRADAXA, increases the risk of thrombotic events. If anticoagulation with PRADAXA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant. (B) SPINAL/EPIDURAL HEMATOMA Epidural or spinal hematomas may occur in patients treated with PRADAXA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. |
Overview
Dabigatran is a direct thrombin inhibitor that is FDA approved for the prophylaxis of stroke and systemic embolism in patients with non-valvular atrial fibrillation. There is a Black Box Warning for this drug as shown here. Common adverse reactions include esophagitis, gastritis, gastroesophageal reflux disease, gastrointestinal hemorrhage, gastrointestinal ulcer, indigestion,bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Atrial fibrillation
- Dosing Information
- Recommended dose for patients creatinine clearance (CrCl) >30 mL/min: 150 mg PO bid
- Recommended dose for patients (CrCl 15-30 mL/min): 75 mg PO bid
- Recommended dose for patients CrCl <15 mL/min or on dialysis: Not provided by FDA
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Postoperative deep vein thrombosis; Prophylaxis
- Developed by: American College of Chest Physicians(ACCP)
- Class of Recommendation: ACCP guidelines classification scheme#Class II:Benefit ≥ Risk
- Level of Evidence:Level B
- Recommendation
- 220 mg or 150 mg PO qd
Atrial fibrillation - Thromboembolic disorder; Prophylaxis
- Developed by: American College of Chest Physicians(ACCP)
- Class of Recommendation: ACCP guidelines classification scheme#Class II:Benefit ≥ Risk
- Level of Evidence:Level B
- Recommendation
- 150 mg PO bid [1]
Postoperative deep vein thrombosis; Prophylaxis
- Developed by: American College of Chest Physicians(ACCP)
- Class of Recommendation: ACCP guidelines classification scheme#Class II:Benefit ≥ Risk
- Level of Evidence:Level B
- Recommendation
Venous thromboembolism
- Developed by: American College of Chest Physicians(ACCP)
- Class of Recommendation: ACCP guidelines classification scheme#Class II:Benefit ≥ Risk
- Level of Evidence:Level B
Pediatric Indications and Dosage
- Safety and effectiveness of PRADAXA in pediatric patients have not been established.
Contraindications
- Active pathological bleeding.
- History of a serious hypersensitivity reaction to PRADAXA (e.g., anaphylactic reaction or anaphylactic shock) .
- Mechanical prosthetic heart valve.
Warnings
Increased Risk of Stroke with Discontinuation of PRADAXA
- Discontinuing PRADAXA in absence of adequate alternative anticoagulation increases the risk of thrombotic events. If PRADAXA must be discontinued for a reason other than pathological bleeding, consider coverage with another anticoagulant.
Risk of Bleeding
- PRADAXA increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue PRADAXA in patients with active pathological bleeding.
- Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). PRADAXA’s anticoagulant activity and half-life are increased in patients with renal impairment.
- A specific reversal agent for dabigatran is not available. Hemodialysis can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited. Activated prothrombin complex concentrates (aPCCs, e.g., FEIBA), or recombinant Factor VIIa, or concentrates of coagulation factors II, IX or X may be considered but their use has not been evaluated in clinical trials. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of dabigatran. Consider administration of platelet concentrates in cases where thrombocytopenia is present or long-acting antiplatelet drugs have been used.
Thromboembolic and Bleeding Events in Patients with Prosthetic Heart Valves
- The safety and efficacy of PRADAXA in patients with bileaflet mechanical prosthetic heart valves was evaluated in the RE-ALIGN trial, in which patients with bileaflet mechanical prosthetic heart valves (recently implanted or implanted more than three months prior to enrollment) were randomized to dose adjusted warfarin or 150, 220, or 300 mg of PRADAXA twice a day. RE-ALIGN was terminated early due to the occurrence of significantly more thromboembolic events (valve thrombosis, stroke, transient ischemic attack, and myocardial infarction) and an excess of major bleeding (predominantly post-operative pericardial effusions requiring intervention for hemodynamic compromise) in the PRADAXA treatment arm as compared to the warfarin treatment arm. These bleeding and thromboembolic events were seen both in patients who were initiated on PRADAXA post-operatively within three days of mechanical bileaflet valve implantation, as well as in patients whose valves had been implanted more than three months prior to enrollment. Therefore, the use of PRADAXA is contraindicated in patients with mechanical prosthetic valves.
- The use of PRADAXA for the prophylaxis of thromboembolic events in patients with atrial fibrillation in the setting of other forms of valvular heart disease, including the presence of a bioprosthetic heart valve, has not been studied and is not recommended.
Effect of P-gp Inducers and Inhibitors on Dabigatran Exposure
- The concomitant use of PRADAXA with P-gp inducers (e.g. rifampin) reduces exposure to dabigatran and should generally be avoided.
- P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
- Consider reducing the dose of PRADAXA to 75 mg twice daily when dronedarone or systemic ketoconazole is coadministered with PRADAXA in patients with moderate renal impairment (CrCl 30-50 mL/min). Avoid use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min).
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
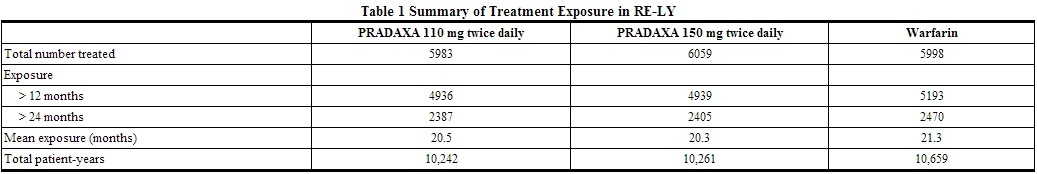
- The RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study provided safety information on the use of two doses of PRADAXA and warfarin. The numbers of patients and their exposures are described in Table 1. Limited information is presented on the 110 mg dosing arm because this dose is not approved.
 |
Drug Discontinuation in RE-LY
- The rates of adverse reactions leading to treatment discontinuation were 21% for PRADAXA 150 mg and 16% for warfarin. The most frequent adverse reactions leading to discontinuation of PRADAXA were bleeding and gastrointestinal events (i.e., dyspepsia, nausea, upper abdominal pain, gastrointestinal hemorrhage, and diarrhea).
Bleeding
- Table 2 shows the number of patients experiencing serious bleeding during the treatment period in the RE-LY study, with the bleeding rate per 800 patient-years (%). Major bleeds fulfilled one or more of the following criteria: bleeding associated with a reduction in hemoglobin of at least 2 grams per deciliter or leading to a transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding). A life-threatening bleed met one or more of the following criteria: fatal, symptomatic intracranial bleed, reduction in hemoglobin of at least 5 grams per deciliter, transfusion of at least 4 units of blood, associated with hypotension requiring the use of intravenous inotropic agents, or necessitating surgical intervention. Intracranial hemorrhage included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
 |
- The risk of major bleeds was similar with PRADAXA 150 mg and warfarin across major subgroups defined by baseline characteristics, with the exception of age, where there was a trend towards a higher incidence of major bleeding on PRADAXA (hazard ratio 1.2, 95% CI: 1.0 to 1.4) for patients ≥75 years of age.
- There was a higher rate of major gastrointestinal bleeds in patients receiving PRADAXA 150 mg than in patients receiving warfarin (1.6% vs. 1.1%, respectively, with a hazard ratio vs. warfarin of 1.5, 95% CI, 1.2 to 1.9), and a higher rate of any gastrointestinal bleeds (6.1% vs. 4.0%, respectively).
Gastrointestinal Adverse Reactions
- Patients on PRADAXA 150 mg had an increased incidence of gastrointestinal adverse reactions (35% vs. 24% on warfarin). These were commonly dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) and gastritis-like symptoms (including GERD, esophagitis, erosive gastritis, gastric hemorrhage, hemorrhagic gastritis, hemorrhagic erosive gastritis, and gastrointestinal ulcer).
Hypersensitivity Reactions
- In the RE-LY study, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in <0.1% of patients receiving PRADAXA.
Postmarketing
- The following adverse reactions have been identified during post approval use of PRADAXA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following adverse reactions have been identified during post approval use of PRADAXA: angioedema, thrombocytopenia, esophageal ulcer.
Drug Interactions
- The concomitant use of PRADAXA with [P-gp inducers] (e.g., rifampin) reduces exposure to dabigatran and should generally be avoided.
- P-gp inhibition and impaired renal function are the major independent factors that result in increased exposure to dabigatran. Concomitant use of P-gp inhibitors in patients with renal impairment is expected to produce increased exposure of dabigatran compared to that seen with either factor alone.
- In patients with moderate renal impairment (CrCl 30-50 mL/min), consider reducing the dose of PRADAXA to 75 mg twice daily when administered concomitantly with the P-gp inhibitor dronedarone or systemic ketoconazole. The use of P-gp inhibitors (verapamil, amiodarone, quinidine, and clarithromycin) does not require a dose adjustment of PRADAXA. These results should not be extrapolated to other P-gp inhibitors.
- The concomitant use of PRADAXA and P-gp inhibitors in patients with severe renal impairment (CrCl 15-30 mL/min) should be avoided.
- There are no adequate and well-controlled studies in pregnant women.
- Dabigatran has been shown to decrease the number of implantations when male and female rats were treated at a dosage of 70 mg/kg (about 2.6 to 3.0 times the human exposure at maximum recommended human dose [MRHD] of 300 mg/day based on area under the curve [AUC] comparisons) prior to mating and up to implantation (gestation Day 6). Treatment of pregnant rats after implantation with dabigatran at the same dose increased the number of dead offspring and caused excess vaginal/uterine bleeding close to parturition. Although dabigatran increased the incidence of delayed or irregular ossification of fetal skull bones and vertebrae in the rat, it did not induce major malformations in rats or rabbits.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Pregnancy Category (AUS): Dabigatran is not included in Australian Drug Evaluation Committee (ADEC) Pregnancy Categories.
Labor and Delivery
- Safety and effectiveness of PRADAXA during labor and delivery have not been studied in clinical trials. Consider the risks of bleeding and of stroke in using PRADAXA in this setting.
- Death of offspring and mother rats during labor in association with uterine bleeding occurred during treatment of pregnant rats from implantation (gestation Day 7) to weaning (lactation Day 21) with dabigatran at a dose of 70 mg/kg (about 2.6 times the human exposure at MRHD of 300 mg/day based on AUC comparisons).
Nursing Mothers
- It is not known whether dabigatran is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when PRADAXA is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness of PRADAXA in pediatric patients have not been established.
Geriatric Use
- Of the total number of patients in the RE-LY study, 82% were 65 and over, while 40% were 75 and over. The risk of stroke and bleeding increases with age, but the risk-benefit profile is favorable in all age groups.
- Use In Renal Impair=No dose adjustment of PRADAXA is recommended in patients with mild or moderate renal impairment. Reduce the dose of PRADAXA in patients with severe renal impairment (CrCl 15-30 mL/min). Dosing recommendations for patients with CrCl <15 mL/min or on dialysis cannot be provided.
- Adjust dose appropriately in patients with renal impairment receiving concomitant P-gp inhibitors.
Gender
- FDA Package Insert for Dabigatran contains no information regarding Gender.
Race
- FDA Package Insert for Dabigatran contains no information regarding Race.
Renal Impairment
- FDA Package Insert for Dabigatran contains no information regarding Renal Impairment.
Hepatic Impairment
- FDA Package Insert for Dabigatran contains no information regarding Hepatic Impairment.
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Dabigatran was not carcinogenic when administered by oral gavage to mice and rats for up to 2 years. The highest doses tested (200 mg/kg/day) in mice and rats were approximately 3.6 and 6 times, respectively, the human exposure at MRHD of 300 mg/day based on AUC comparisons.
- Dabigatran was not mutagenic in in vitro tests, including bacterial reversion tests, mouse lymphoma assay and chromosomal aberration assay in human lymphocytes, and thein vivo micronucleus assay in rats.
- In the rat fertility study with oral gavage doses of 15, 70, and 200 mg/kg, males were treated for 29 days prior to mating, during mating up to scheduled termination, and females were treated 15 days prior to mating through gestation Day 6. No adverse effects on male or female fertility were observed at 200 mg/kg or 9 to 12 times the human exposure at MRHD of 300 mg/day based on AUC comparisons. However, the number of implantations decreased in females receiving 70 mg/kg, or 3 times the human exposure at MRHD based on AUC comparisons.
Immunocompromised Patients
- FDA Package Insert for Dabigatran contains no information regarding Immunocompromised Patients.
Miscellaneous
- FDA Package Insert for Dabigatran contains no information regarding Miscellaneous.
Administration and Monitoring
Administration
- Oral
Instructions to Patients
- Instruct patients to swallow the capsules whole. PRADAXA should be taken with a full glass of water. Breaking, chewing, or emptying the contents of the capsule can result in increased exposure.
- If a dose of PRADAXA is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. The dose of PRADAXA should not be doubled to make up for a missed dose.
Converting from or to Warfarin
- When converting patients from warfarin therapy to PRADAXA, discontinue warfarin and start PRADAXA when the INR is below 2.0.
- When converting from PRADAXA to warfarin, adjust the starting time of warfarin based on creatinine clearance as follows:
- For CrCl ≥50 mL/min, start warfarin 3 days before discontinuing PRADAXA.
- For CrCl 30-50 mL/min, start warfarin 2 days before discontinuing PRADAXA.
- For CrCl 15-30 mL/min, start warfarin 1 day before discontinuing PRADAXA.
- For CrCl <15 mL/min, no recommendations can be made.
- Because PRADAXA can increase INR, the INR will better reflect warfarin’s effect only after PRADAXA has been stopped for at least 2 days.
Converting from or to Parenteral Anticoagulants
- For patients currently receiving a parenteral anticoagulant, start PRADAXA 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (e.g., intravenous unfractionated heparin).
- For patients currently taking PRADAXA, wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of PRADAXA before initiating treatment with a parenteral anticoagulant.
Surgery and Interventions
- If possible, discontinue PRADAXA 1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required.
- If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention.
- Monitoring=INR is relatively insensitive to the exposure to dabigatran and cannot be interpreted the same way as used for warfarin monitoring.
- IV Compat=FDA Package Insert for Metoprolol tartrate contains no information regarding Black Box Warning.
- Overdose=Accidental overdose may lead to hemorrhagic complications. There is no reversal agent for dabigatran. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with PRADAXA, and investigate the source of bleeding. Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy
IV Compatibility
- FDA Package Insert for Dabigatran contains no information regarding IV Compatibility.
Overdosage
- Accidental overdose may lead to hemorrhagic complications. There is no reversal agent for dabigatran. In the event of hemorrhagic complications, initiate appropriate clinical support, discontinue treatment with PRADAXA, and investigate the source of bleeding. Dabigatran is primarily eliminated by the kidneys with a low plasma protein binding of approximately 35%. Hemodialysis can remove dabigatran; however, data supporting this approach are limited. Using a high-flux dialyzer, blood flow rate of 200 mL/min, and dialysate flow rate of 700 mL/min, approximately 49% of total dabigatran can be cleared from plasma over 4 hours. At the same dialysate flow rate, approximately 57% can be cleared using a dialyzer blood flow rate of 300 mL/min, with no appreciable increase in clearance observed at higher blood flow rates. Upon cessation of hemodialysis, a redistribution effect of approximately 7% to 15% is seen. The effect of dialysis on dabigatran’s plasma concentration would be expected to vary based on patient specific characteristics. Measurement of aPTT or ECT may help guide therapy.
Pharmacology
Mechanism of Action
- Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors. Because thrombin (serine protease) enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus. Both free and clot-bound thrombin, and thrombin-induced platelet aggregation are inhibited by the active moieties.
Structure
{{drugbox2
| verifiedrevid = 407639691
| drug_name = Dabigatran etexilate
| IUPAC_name = Ethyl 3-{[(2-{[(4-{N'-hexyloxycarbonyl carbamimidoyl}phenyl)amino]methyl}-1-
methyl-1H-benzimidazol-5-yl)carbonyl] (pyridin-2-yl-amino)propanoate
| image = Dabigatran etexilate structure.png
| width = 135
| image2 = Dabigatran etexilate ball-and-stick.png
| width2 = 250
| tradename = Pradaxa,Prazaxa
| ChemSpiderID_Ref =
| ChemSpiderID = 4948999
| InChI = 1/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44)
| smiles = O=C(OCC)CCN(c1ncccc1)C(=O)c4ccc2c(nc(n2C)CNc3ccc(C(=N\C(=O)OCCCCCC)\N)cc3)c4
| InChIKey = KSGXQBZTULBEEQ-UHFFFAOYAL
| ChEMBL_Ref =
| ChEMBL = 539697
| StdInChI_Ref =
| StdInChI = 1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44)
| StdInChIKey_Ref =
| StdInChIKey = KSGXQBZTULBEEQ-UHFFFAOYSA-N
| CAS_number = 211915-06-9
| ATC_prefix = B01
| ATC_suffix = AE07
| ATC_supplemental=
| PubChem = 6445226
| DrugBank = DB06695
| chemical_formula =
| C=34 | H=41 | N=7 | O=5
| molecular_weight = 627.734 g/mol
| specific_rotation =
| sec_combustion =
| bioavailability = 3–7%[2]
| protein_bound = 35%[2]
| metabolism =
| elimination_half-life = 12–17 hours[2]
| excretion =
| pregnancy_AU =
| pregnancy_US = C
| pregnancy_category =
| legal_AU =
| legal_CA = Schedule VI
| legal_UK = POM
| legal_US = Rx-only
| legal_status =
| dependency_liability =
| routes_of_administration = oral
| licence_EU = Pradaxa
| licence_US = Dabigatran
| MedlinePlus = a610024
}}
Pharmacodynamics
- At recommended therapeutic doses, dabigatran etexilate prolongs the coagulation markers such as aPTT, ECT, and TT. INR is relatively insensitive to the exposure to dabigatran and cannot be interpreted the same way as used for warfarin monitoring.
- The aPTT test provides an approximation of PRADAXA’s anticoagulant effect. The average time course for effects on aPTT, following approved dosing regimens in patients with various degrees of renal impairment is shown in Figure 1. The curves represent mean levels without confidence intervals; variations should be expected when measuring aPTT. While advice cannot be provided on the level of recovery of aPTT needed in any particular clinical setting, the curves can be used to estimate the time to get to a particular level of recovery, even when the time since the last dose of PRADAXA is not precisely known. In the RE-LY trial, the median (10th to 90th percentile) trough aPTT in patients receiving the 150 mg dose was 52 (40 to 76) seconds.
- Simulations based on PK data from a study in subjects with renal impairment and PK/aPTT relationships derived from the RE-LY study; aPTT prolongation in RE-LY was measured centrally in citrate plasma using PTT Reagent Roche Diagnostics GmbH, Mannheim, Germany. There may be quantitative differences between various established methods for aPTT assessment.
- The degree of anticoagulant activity can also be assessed by the ecarin clotting time (ECT). This test is a more specific measure of the effect of dabigatran than activated partial thromboplastin time (aPTT). In the RE-LY trial, the median (10th to 90th percentile) trough ECT in patients receiving the 150 mg dose was 63 (44 to 103) seconds.
Cardiac Electrophysiology
- No prolongation of the QTc interval was observed with dabigatran etexilate at doses up to 600 mg.
Pharmacokinetics
- Dabigatran etexilate mesylate is absorbed as the dabigatran etexilate ester. The ester is then hydrolyzed, forming dabigatran, the active moiety. Dabigatran is metabolized to four different acyl glucuronides and both the glucuronides and dabigatran have similar pharmacological activity. Pharmacokinetics described here refer to the sum of dabigatran and its glucuronides. Dabigatran displays dose-proportional pharmacokinetics in healthy subjects and patients in the range of doses from 10 to 400 mg.
Distribution
- Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran’s accumulation factor is approximately two.
Elimination
- Dabigatran is eliminated primarily in the urine. Renal clearance of dabigatran is 80% of total clearance after intravenous administration. After oral administration of radiolabeled dabigatran, 7% of radioactivity is recovered in urine and 86% in feces. The half-life of dabigatran in healthy subjects is 12 to 17 hours.
Metabolism
- After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma.
Renal Impairment
- An open, parallel-group single-center study compared dabigatran pharmacokinetics in healthy subjects and patients with mild to moderate renal impairment receiving a single dose of PRADAXA 150 mg. Exposure to dabigatran increases with severity of renal function impairment (Table 3). Similar findings were observed in the RE-LY trial.
| thumb|600px|left |
Hepatic Impairment
- Administration of PRADAXA in patients with moderate hepatic impairment (Child-Pugh B) showed a large inter-subject variability, but no evidence of a consistent change in exposure or pharmacodynamics.
Drug Interactions
- Impact of Other Drugs on Dabigatran
P-gp Inducers
- Rifampin: Rifampin 600 mg once daily for 7 days followed by a single dose of dabigatran decreased its AUC and Cmax by 66% and 67%, respectively. By Day 7 after cessation of rifampin treatment, dabigatran exposure was close to normal.
P-gp Inhibitors
- In studies with the P-gp inhibitors ketoconazole, amiodarone, verapamil, and quinidine, the time to peak, terminal half-life, and mean residence time of dabigatran were not affected. Any observed changes in Cmax and AUC are described below.
Dronedarone: Simultaneous administration of dabigatran etexilate and dronedarone (administered once or twice daily) increases exposure to dabigatran by 70 to 140% compared to dabigatran alone. The increase in exposure is only 30 to 60% higher compared to dabigatran alone when dronedarone is administered 2 hours after dabigatran etexilate.
Ketoconazole: Systemic ketoconazole increased dabigatran AUC and Cmax values by 138% and 135%, respectively, after a single dose of 400 mg, and 153%, and 149%, respectively, after multiple daily doses of 400 mg.
Verapamil: When dabigatran etexilate was coadministered with oral verapamil, the Cmax and AUC of dabigatran were increased. The extent of increase depends on the formulation of verapamil and timing of administration. If verapamil is present in the gut when dabigatran is taken, it will increase exposure to dabigatran with the greatest increase observed when a single dose of immediate-release verapamil is given one hour prior to dabigatran (AUC increased by a factor of 2.4). If verapamil is given 2 hours after dabigatran, the increase in AUC is negligible. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received verapamil.
Amiodarone: When dabigatran etexilate was coadministered with a single 600 mg oral dose of amiodarone, the dabigatran AUC and Cmax increased by 58% and 50%, respectively. The increase in exposure was mitigated by a 65% increase in the renal clearance of dabigatran in the presence of amiodarone. The increase in renal clearance may persist after amiodarone is discontinued because of amiodarone’s long half-life. In the population pharmacokinetics study from RE-LY, no important changes in dabigatran trough levels were observed in patients who received amiodarone.
Quinidine: Quinidine was given as 200 mg dose every 2 hours up to a total dose of 1000 mg. Dabigatran etexilate was given over 3 consecutive days, the last evening dose on Day 3 with or without quinidine pre-dosing. Concomitant quinidine administration increased dabigatran’s AUC and Cmax by 53% and 56%, respectively.
Clarithromycin: Coadministered clarithromycin had no impact on the exposure to dabigatran.
Other Drugs
Clopidogrel: When dabigatran etexilate was given concomitantly with a loading dose of 300 mg or 600 mg clopidogrel, the dabigatran AUC and Cmax increased by approximately 30% and 40%, respectively. The concomitant administration of dabigatran etexilate and clopidogrel resulted in no further prolongation of capillary bleeding times compared to clopidogrel monotherapy. When comparing combined treatment and the respective mono-treatments, the coagulation measures for dabigatran’s effect (aPTT, ECT, and TT) remained unchanged, and inhibition of platelet aggregation (IPA), a measurement of clopidogrel’s effect, remained unchanged.
Enoxaparin: Enoxaparin 40 mg given subcutaneously for 3 days with the last dose given 24 hours before a single dose of PRADAXA had no impact on the exposure to dabigatran or the coagulation measures aPTT, ECT, or TT.
Diclofenac, Ranitidine, and Digoxin:None of these drugs alters exposure to dabigatran.
In RE-LY, dabigatran plasma samples were also collected. The concomitant use of proton pump inhibitors, H2 antagonists, and digoxin did not appreciably change the trough concentration of dabigatran.
Impact of Dabigatran on Other Drugs
- In clinical studies exploring CYP3A4, CYP2C9, P-gp and other pathways, dabigatran did not meaningfully alter the pharmacokinetics of amiodarone, atorvastatin, clarithromycin, diclofenac, clopidogrel, digoxin, pantoprazole, or ranitidine.
Nonclinical Toxicology
- FDA Package Insert for Dabigatran contains no information regarding Nonclinical Toxicology.
Clinical Studies
- The clinical evidence for the efficacy of PRADAXA was derived from RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy), a multi-center, multi-national, randomized parallel group trial comparing two blinded doses of PRADAXA (110 mg twice daily and 150 mg twice daily) with open-label warfarin (dosed to target INR of 2 to 3) in patients with non-valvular, persistent, paroxysmal, or permanent atrial fibrillation and one or more of the following additional risk factors:
- Previous stroke, transient ischemic attack (TIA), or systemic embolism
- Left ventricular ejection fraction <40%
- Symptomatic heart failure, ≥ New York Heart Association Class 2
- Age ≥75 years
- Age ≥65 years and one of the following: diabetes mellitus, coronary artery disease (CAD), or hypertension
- The primary objective of this study was to determine if PRADAXA was non-inferior to warfarin in reducing the occurrence of the composite endpoint, stroke (ischemic and hemorrhagic) and systemic embolism. The study was designed to ensure that PRADAXA preserved more than 50% of warfarin’s effect as established by previous randomized, placebo-controlled trials of warfarin in atrial fibrillation. Statistical superiority was also analyzed.
- A total of 18,113 patients were randomized and followed for a median of 2 years. The patient’s mean age was 71.5 years and the mean CHADS2 score was 2.1. The patient population was 64% male, 70% Caucasian, 16% Asian, and 1% black. Twenty percent of patients had a history of a stroke or TIA and 50% were Vitamin K antagonist (VKA) naïve, defined as less than 2 months total lifetime exposure to a VKA. Thirty-two percent of the population had never been exposed to a VKA. Concomitant diseases of patients in this trial included hypertension 79%, diabetes 23%, and CAD 28%. At baseline, 40% of patients were on aspirin and 6% were on clopidogrel. For patients randomized to warfarin, the mean percentage of time in therapeutic range (INR 2 to 3) was 64%.
- Relative to warfarin and to PRADAXA 110 mg twice daily, PRADAXA 150 mg twice daily significantly reduced the primary composite endpoint of stroke and systemic embolism (see Table 4 and Figure 2).
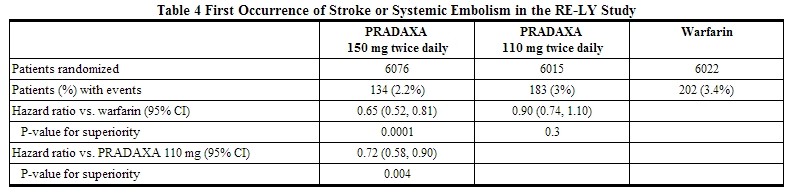 |
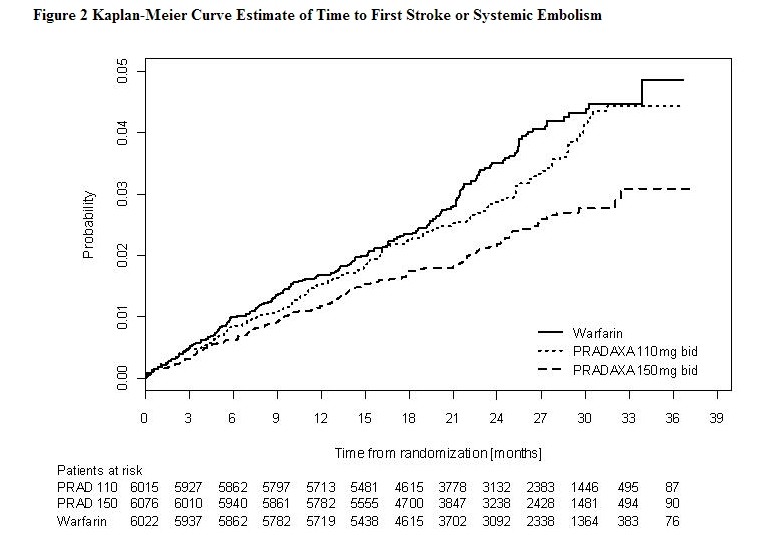 |
- The contributions of the components of the composite endpoint, including stroke by subtype, are shown in Table 5. The treatment effect was primarily a reduction in stroke. PRADAXA 150 mg twice daily was superior in reducing ischemic and hemorrhagic strokes relative to warfarin.
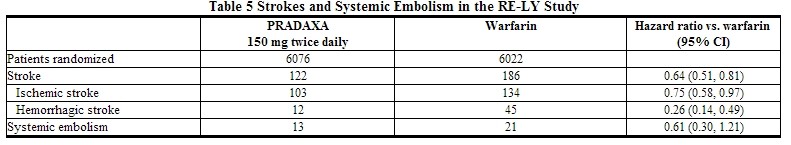 |
- In the RE-LY trial, the rate of all-cause mortality was lower on dabigatran 150 mg than on warfarin (3.6% per year versus 4.1% per year). The rate of vascular death was lower on dabigatran 150 mg compared to warfarin (2.3% per year versus 2.7% per year). Non-vascular death rates were similar in the treatment arms.
- The efficacy of PRADAXA 150 mg twice daily was generally consistent across major subgroups (see Figure 3).
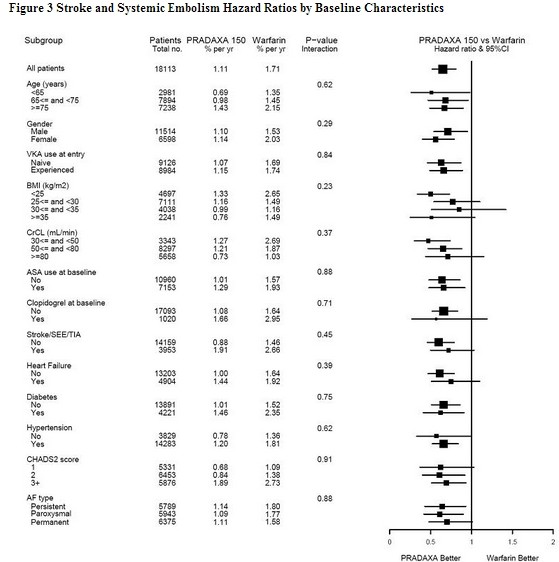 |
- In RE-LY, a higher rate of clinical myocardial infarction was reported in patients who received PRADAXA (0.7 per 100 patient-years for 150 mg dose) than in those who received warfarin (0.6).
How supplied
- PRADAXA 75 mg capsules have a cream-colored opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R75". The color of the imprinting is black. The capsules are supplied in the packages listed:
- NDC 0597-0149-54 Unit of use bottle of 60 capsules
- NDC 0597-0149-60 Blister package containing 60 capsules (10 x 6 capsule blister cards)
- PRADAXA 150 mg capsules have a light blue opaque cap imprinted with the Boehringer Ingelheim company symbol and a cream-colored opaque body imprinted with "R150". The color of the imprinting is black. The capsules are supplied in the packages listed:
- NDC 0597-0135-54 Unit of use bottle of 60 capsules
- NDC 0597-0135-60 Blister package containing 60 capsules (10 x 6 capsule blister cards)
Bottles
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Once opened, the product must be used within 4 months. Keep the bottle tightly closed. Store in the original package to protect from moisture.
Blisters
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Store in the original package to protect from moisture.
Images
Drug Images
Package and Label Display Panel
 |
 |
 |
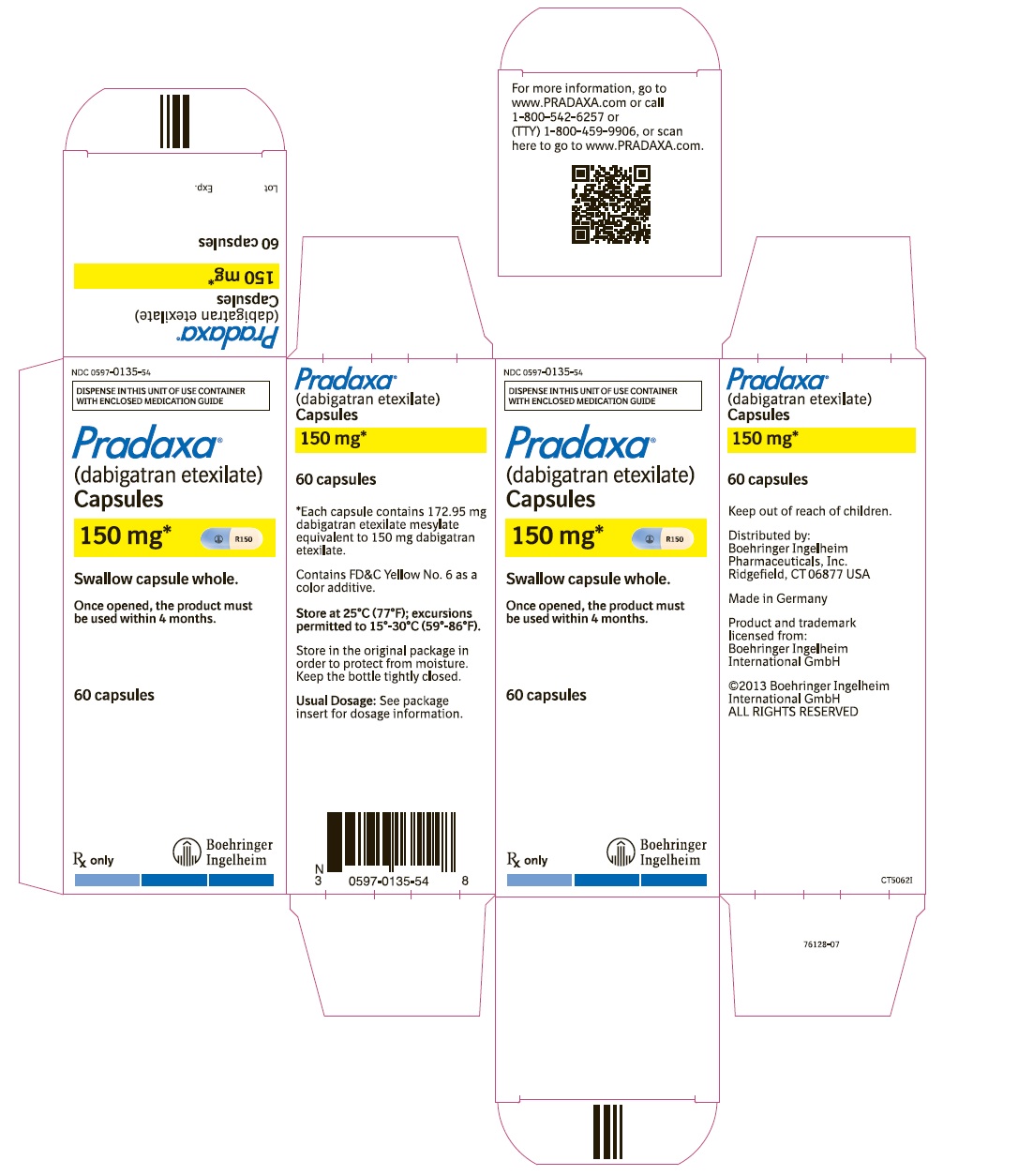 |
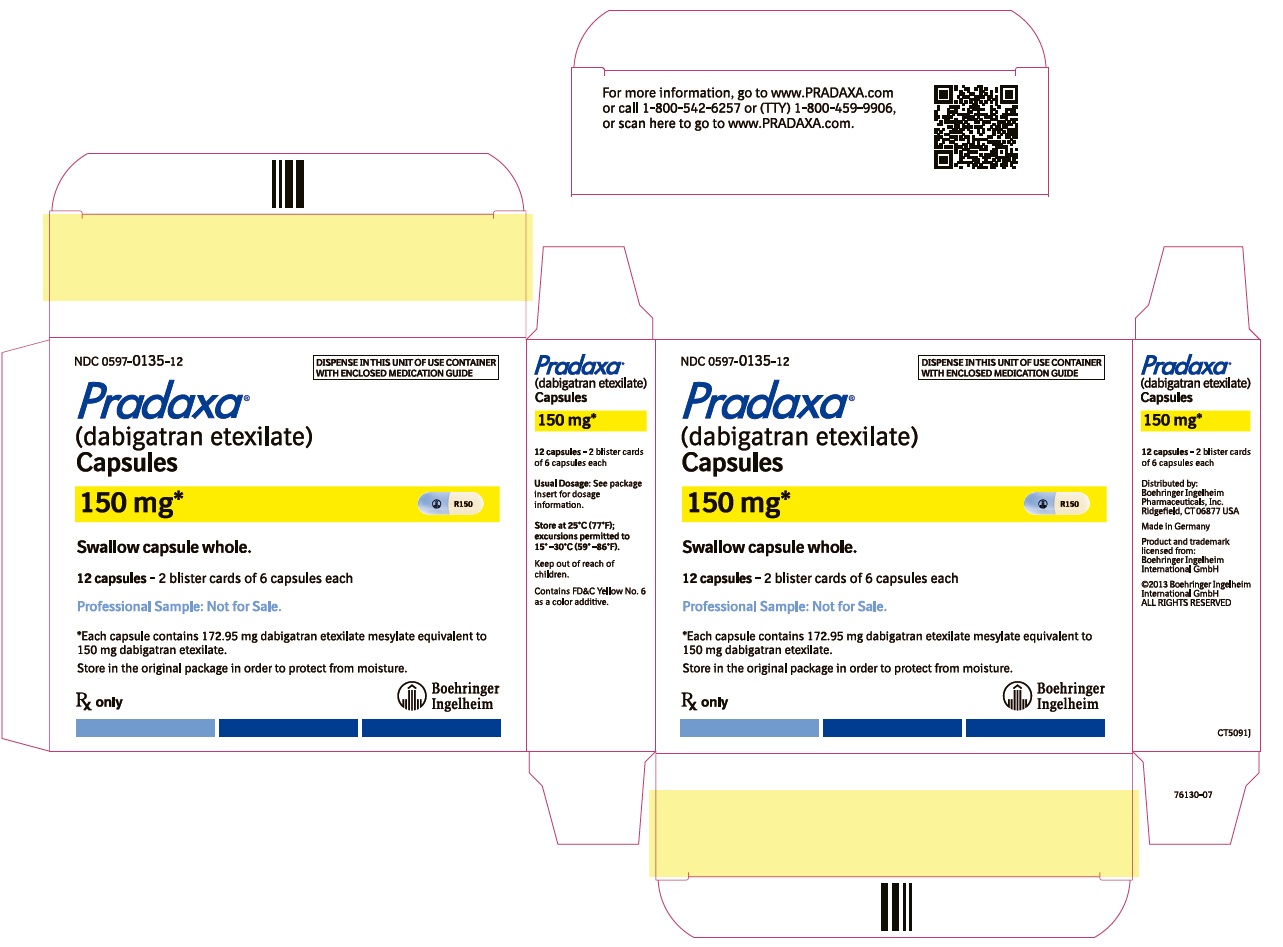 |
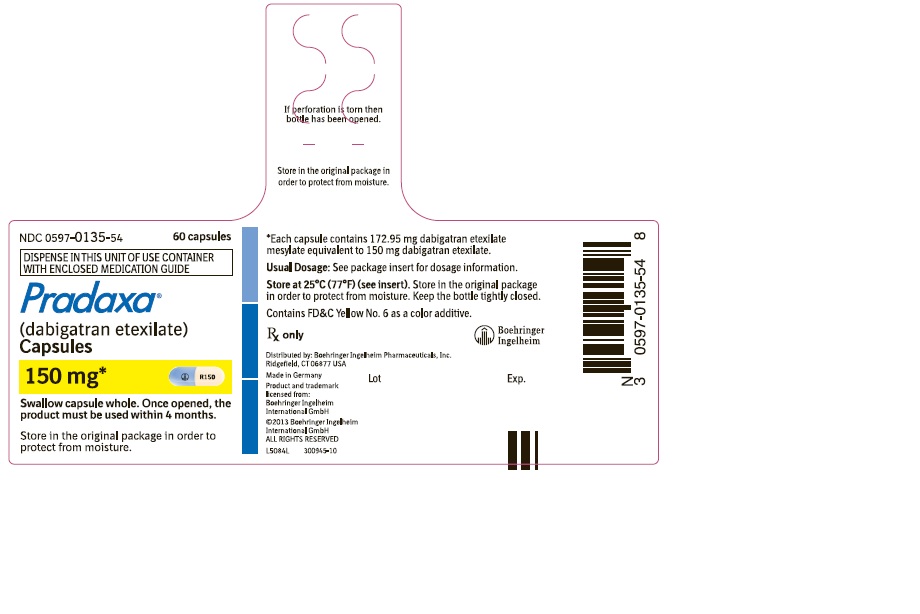 |
 |
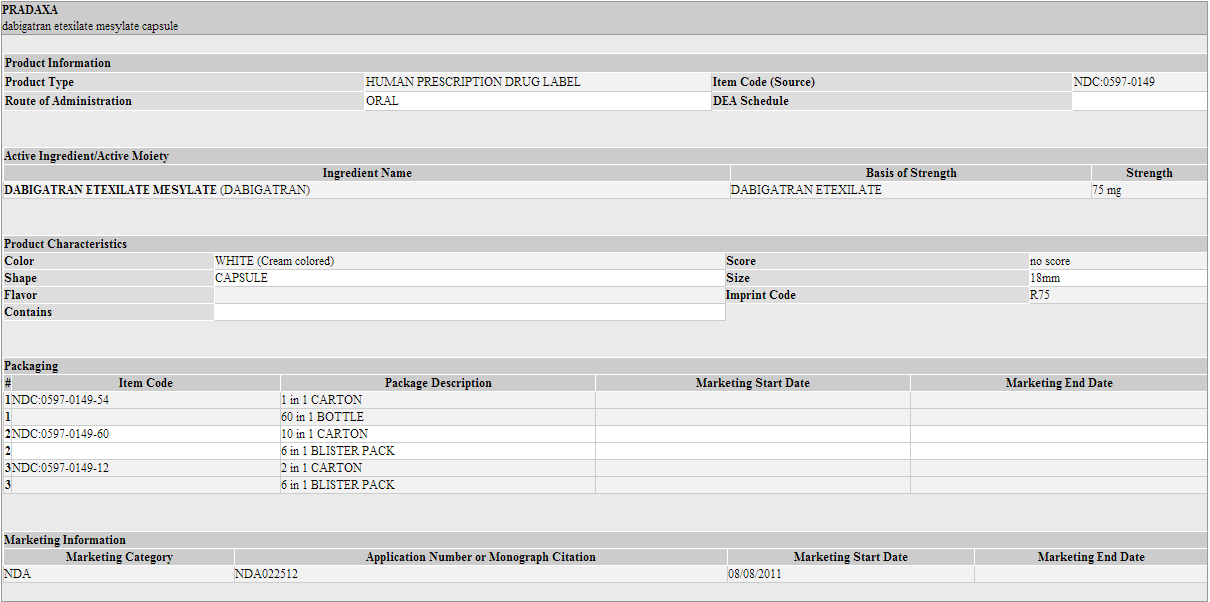 |
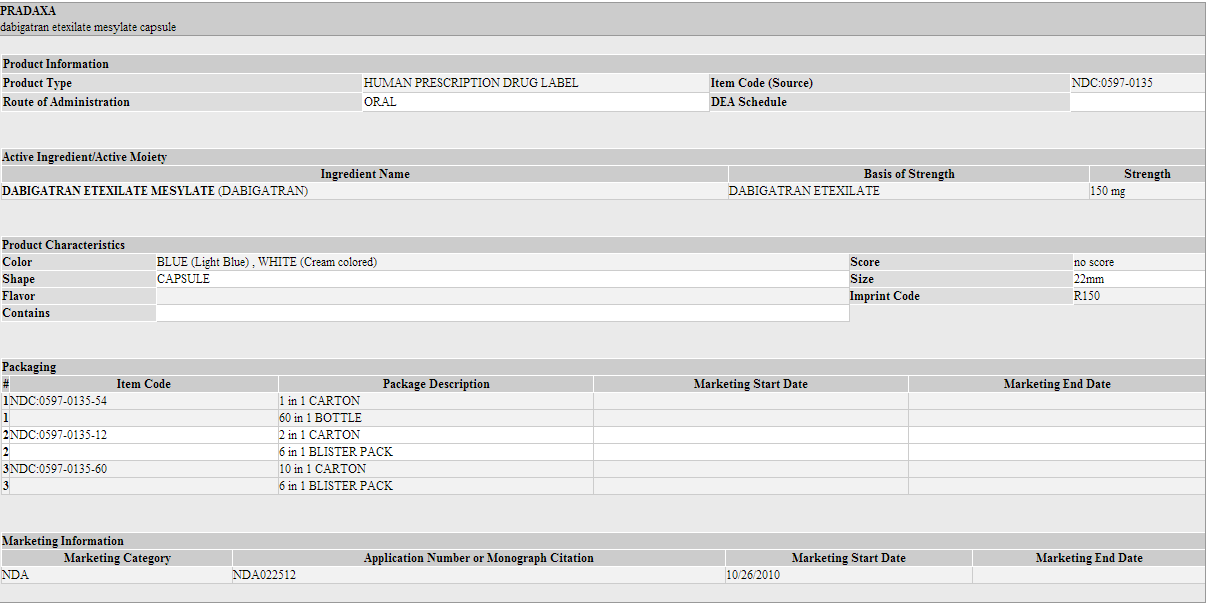 |
Patient Information
Patient Information from FDA
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instructions for Patients
- Tell patients to take PRADAXA exactly as prescribed.
- Remind patients not to discontinue PRADAXA without talking to the health care provider who prescribed it.
- Keep PRADAXA in the original bottle to protect from moisture. Do not put PRADAXA in pill boxes or pill organizers.
- When more than one bottle is dispensed to the patient, instruct them to open only one bottle at a time.
- Instruct patient to remove only one capsule from the opened bottle at the time of use. The bottle should be immediately and tightly closed.
- Advise patients not to chew or break the capsules before swallowing them and not to open the capsules and take the pellets alone.
- Advise patients that the capsule should be taken with a full glass of water.
Bleeding
- Inform patients that they may bleed more easily, may bleed longer, and should call their health care provider for any signs or symptoms of bleeding.
- Instruct patients to seek emergency care right away if they have any of the following, which may be a sign or symptom of serious bleeding:
- Unusual bruising (bruises that appear without known cause or that get bigger)
- Pink or brown urine
- Red or black, tarry stools
- Coughing up blood
- Vomiting blood, or vomit that looks like coffee grounds
- Instruct patients to call their health care provider or to get prompt medical attention if they experience any signs or symptoms of bleeding:
- Pain, swelling or discomfort in a joint
- Headaches, dizziness, or weakness
- Reoccurring nose bleeds
- Unusual bleeding from gums
- Bleeding from a cut that takes a long time to stop
- Menstrual bleeding or vaginal bleeding that is heavier than normal
Gastrointestinal Adverse Reactions
- Instruct patients to call their health care provider if they experience any signs or symptoms of dyspepsia or gastritis:
- Dyspepsia (upset stomach), burning, or nausea
- Abdominal pain or discomfort
- Epigastric discomfort, GERD (gastric indigestion)
Invasive or Surgical Procedures
- Instruct patients to inform their health care provider that they are taking PRADAXA before any invasive procedure (including dental procedures) is scheduled.
Concomitant Medications
- Ask patients to list all prescription medications, over-the-counter medications, or dietary supplements they are taking or plan to take so their health care provider knows about other treatments that may affect bleeding risk (e.g., aspirin or NSAIDs) or dabigatran exposure (e.g., dronedarone or systemic ketoconazole).
Prosthetic Heart Valves
- Instruct patients to inform their health care provider if they will have or have had surgery to place a prosthetic heart valve.
Patient Information from NLM
- For patient information about dabigatran from NLM, click here.
Precautions with Alcohol
- Alcohol-Dabigatran interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Pradaxa®
Look-Alike Drug Names
Drug Shortage Status
Price
References
- ↑ Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S et al. (2012) Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141 (2 Suppl):e278S-325S. DOI:10.1378/chest.11-2404 PMID: 22315265
- ↑ 2.0 2.1 2.2 "Pradaxa Full Prescribing Information" (PDF). Boehringer Ingelheim. Retrieved October 2010. Check date values in:
|accessdate=(help)