Coronary artery bypass surgery surgical procedure
|
Coronary Artery Bypass Surgery Microchapters | |
|
Pathophysiology | |
|---|---|
|
Diagnosis | |
|
Treatment | |
|
Perioperative Monitoring | |
|
Surgical Procedure | |
|
Special Scenarios | |
|
Coronary artery bypass surgery surgical procedure On the Web | |
|
Coronary artery bypass surgery surgical procedure in the news | |
|
Blogs on Coronary artery bypass surgery surgical procedure|- |
|
|
Directions to Hospitals Performing Coronary artery bypass surgery surgical procedure | |
|
Risk calculators for Coronary artery bypass surgery surgical procedure | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Associate Editors-in-Chief: Cafer Zorkun, M.D., Ph.D. [3],Mohammed A. Sbeih, M.D. [4]
The Traditional coronary artery bypass grafting procedure (simplified)
- The patient is brought to the operating room and moved onto the operating table.
- An anesthetist places a variety of intravenous lines, often including a pulmonary artery catheter and injects an induction agent (usually propofol) to render the patient unconscious and to anesthetize the patient.
- An endotracheal tube is inserted and secured by the anesthetist or a respiratory therapist and mechanical ventilation is started.
- The chest is opened via a median sternotomy and the heart is examined by the surgeon.
- The grafts are harvested - frequent conduits are the internal thoracic arteries, radial arteries and saphenous veins.
- The surgeon stops the heart and initiates cardiopulmonary bypass; or in the case of "off-pump" surgery, places devices to stabilize the heart.
- One end of each graft is sewn onto the coronary arteries beyond the blockages and the other end is attached to the aorta.
- The heart is restarted; or in "off-pump" surgery, the stabilizing devices are removed. In some cases, the aorta is partially occluded by a C shaped clamp, the heart is restarted and suturing of the grafts to the aorta is done in this partially occluded section of the aorta while the heart is beating. This reduces time spent on the heart lung machine.
- The sternum is wired together and the incisions are sutured closed.
- The patient is moved to the intensive care unit (ICU) to recover. After awakening and stabilizing in the ICU (approximately 1 day), the patient is transferred to the cardiac surgery unit until ready to go home (approximately 4 days).
Minimally invasive CABG
Alternate methods of minimally invasive coronary artery bypass surgery have been developed in recent times. Off-pump coronary artery bypass surgery (OPCAB) is a technique of performing bypass surgery without the use of cardiopulmonary bypass (the heart-lung machine). Futher refinements to OPCAB have resulted in Minimally invasive direct coronary artery bypass surgery (MIDCAB) which is a technique of performing bypass surgery through a 5 to 10 cm incision. People with multi-vessel coronary disease and desire a minimally invasive approach to surgery may be eligible for hybrid bypass. A hybrid approach combines coronary bypass (using the MIDCAB approach) and coronary stenting.
Conduits used for bypass
The choice of conduits (arteries and/or veins from elsewhere in the body) to bypass the blockages is dependent on the surgeon and institution. Typically, the left internal thoracic artery (LITA) (also referred to as the left internal mammary artery or LIMA) is grafted to the Left Anterior Descending artery and a combination of other arteries and veins is used for other coronary arteries. The right internal thoracic artery (RITA), the great saphenous vein from the leg and the radial artery from the forearm are frequently used. The right gastroepiploic artery from the stomach is infrequently used given the difficult mobilization from the abdomen.
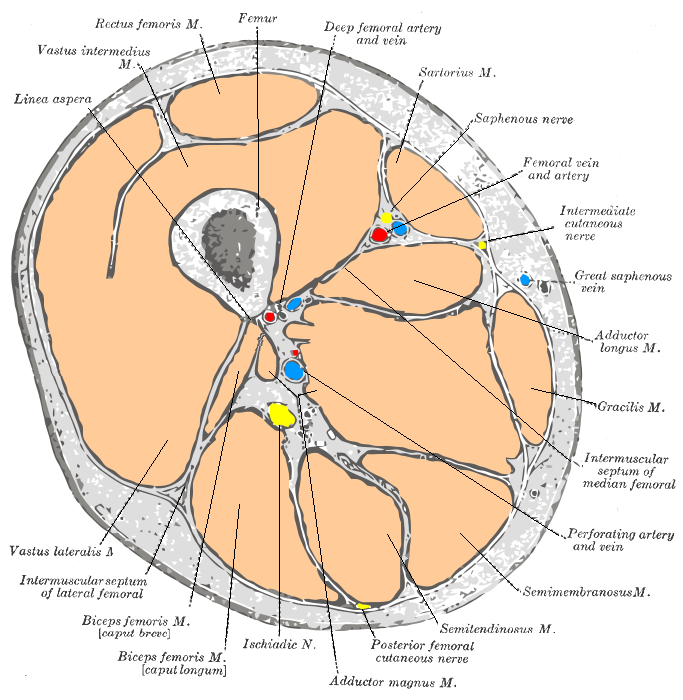
Saphenous vein anatomy
The great saphenous vein (GSV) is frequently used as a conduit for CABG. It originates from where the dorsal vein of the first digit (the large toe) merges with the dorsal venous arch of the foot.
After passing anterior to the medial malleolus (where it often can be visualized and palpated), it runs up the medial side of the leg. At the knee, it runs over the posterior border of the medial epicondyle of the femur bone.
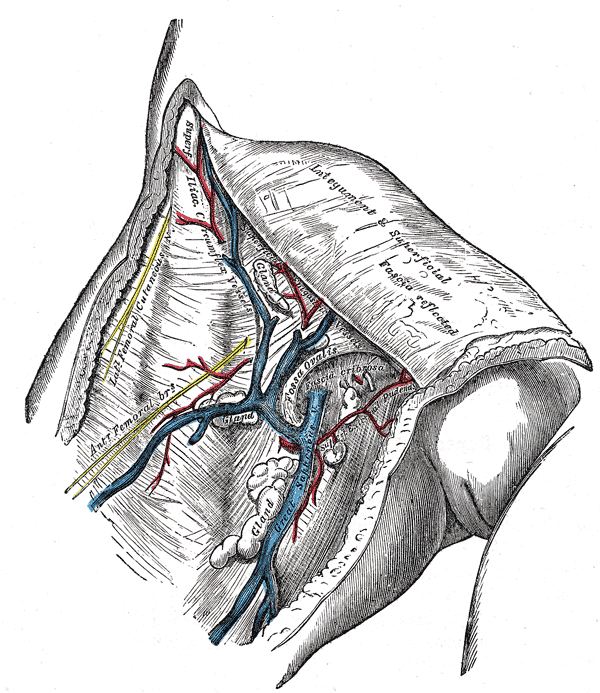
The great saphenous vein then courses laterally to lie on the anterior surface of the thigh before entering an opening in the fascia lata called the saphenous opening. It joins with the femoral vein in the region of the femoral triangle at the saphenofemoral junction.
The small saphenous vein (also lesser saphenous vein) originates where the dorsal vein from the fifth digit (smallest toe) merges with the dorsal venous arch of the foot, which attaches to the great saphenous vein. It is considered a superficial vein and is subcutaneous (just under the skin). From its origin, it courses around the lateral aspect of the foot (inferior and posterior to the lateral malleolus) and runs along the posterior aspect of the leg (with the sural nerve), passes between the heads of the gastrocnemius muscle, and drains into the popliteal vein, approximately at or above the level of the knee joint.
-
Cross-section through the middle of the thigh.
-
Cross-section through middle of leg.
-
The great saphenous vein and landmarks along its course
-
The great saphenous vein and its tributaries at the fossa ovalis in the groin.
-
Small saphenous vein and its tributaries.
Saphenous vein harvesting
The saphenous vein can be harvested by either direct visualization or via an endoscopic approach. Veins that are used either have their valves removed or are turned around so that the valves in them do not occlude blood flow in the graft. The technique of saphenous vein harvesting may influence later SVG patency. The process of harvesting the vein and pressure testing the vein for a leak may damage the endothelium.[1][2]
The endoscopic approach has been associated with lower rates of wound infection, greater patient satisfaction, and earlier mobilization.[3][4][5][6] One small randomized study of 144 patients showed no difference in histologic findings between the traditional and endoscopic techniques.[3]In another small study of 40 patients randomized to the two techniques, no difference was seen in angiographic patency at 3 months.[6]Another small randomized study of 144 patients who returned for angiography demonstrated an occlusion rate of 21.7% for the endoscopic approach vs 17.6% for the open approach.[5] However, non- randomized data from a much larger multicenter study does suggest that endoscopic harvesting may be associated with higher rates of failure and adverse events such as death and MI.[4]
Complications associated with saphenous vein harvesting include the following:
- Saphenous nerve injury
- Infection at incision sites or sepsis.
- Deep vein thrombosis (DVT)
- Keloid scarring
- Chronic pain at incision sites
Videos on spahenous vein graft harvesting
{{#ev:youtube|VbdE6JWdY1s}}
{{#ev:youtube|QthyR0bTHzc}}
{{#ev:youtube|sV-qE2SIkJU}}
Conduit nomenclature
The terms single bypass, double bypass, triple bypass, quadruple bypass and quintuple bypass refer to the number of coronary arteries bypassed in the procedure.
In other words, a double bypass means two coronary arteries are bypassed (e.g. the left anterior descending (LAD) coronary artery and right coronary artery (RCA)); a triple bypass means three vessels are bypassed (e.g. LAD, RCA, left circumflex artery (LCX)); a quadruple bypass means four vessels are bypassed (e.g. LAD, RCA, LCX, first diagonal artery of the LAD) while quintuple means five. Less commonly more than four coronary arteries may be bypassed.
A greater number of bypasses does not imply a person is "sicker," nor does a lesser number imply a person is "healthier."[7] A person with a large amount of coronary artery disease (CAD) may receive fewer bypass grafts owing to the lack of suitable "target" vessels.
A patient with a single stenosis ("narrowing") of the left main coronary artery often requires only two bypasses (to the LAD and the LCX). However, depending upon the anatomy, grafts may also need to be placed to a large diagonal artery, or to additional large obtuse marginal branches.
Assessment of target vessels for saphenous vein grafting
A coronary artery may be unsuitable for bypass grafting for the following reasons:
- Size: If the native target artery is small (< 1 mm or < 1.5 mm depending on surgeon preference)
- Location: Some distal locations of the native target artery may not be accessible, or a conduit may not reach the far down the native artery.
- Native artery calcification: Heavily calcified native arteries are sometimes technically not amenable to anastamosis of a conduit.
- Diffuse disease: The native artery may not have a section of vessel that has minimal disease where a conduit can be grafted to.
- The native artery lies in the heart muscle or is intramyocardial: In this scenario the native coronary artery is located within the heart muscle rather than on the surface of the heart and a graft cannot be attached to it.
Although the cardiothoracic surgeon reviews the coronary angiogram prior to surgery and identifies the lesions (or "blockages") in the coronary arteries and will estimate the number of bypass grafts prior to surgery, the final decision is made in the operating room based upon the direct examination of the heart and the suitability of the native target vessel for bypassing.
Pathophysiology of saphenous vein graft disease
The pathophysiology of aortocoronary saphenous vein graft disease has classically been divided into three components:[8]
- Early degeneration in the first month due to thrombosis
- Mid course degeneration from month one to one year due to intimal hyperplasia
- Late degeneration due to atherosclerosis
Early failure of saphenous vein grafts
While thrombosis predominates during this period, early failure of saphenous vein grafts can be precipitated by a variety of factors.
Technical failures
This is due to a technical failure at the site where the conduit is sutured to the aorta proximately or to the native target vessel distally. This technical failure then leads to thrombosis of the conduit. This failure can in some cases be treated by emergency re-operation or percutaneously by angioplasty and stenting. Care must be taken during the percutaneous approach to assure that the sutures are not disrupted and that there is not a rupture at the site of the anastomosis.
No reflow downstream in the myocardium
Despite restoration of into or improved flow down the epicardial artery, myocardial edema, embolization, and capillary blistering may result in impaired perfusion into the myocardium. This in turn may lead to poor flow through the graft, and subsequent thrombosis.
Midcourse failures of saphenous vein grafts
Midcourse degeneration from one month to one year is classically described as being due to intimal hyperplasia.[9]The process is complex and the response of the SVG wall is likely biphasic. Days to weeks after CABG, there is an increase in the area of both the intima (due to smooth muscle cell proliferation) [10]and the adventitia (due to fibroblast proliferation)[11]. After the first 4 to 6 weeks, the SVGs then undergo "negative remodeling", that is to say there is a loss in the total vessel diamter. In one study using CT angiography, there was a mean loss of SVG lumen diameter of 9% (a decrease from 3.69 mm to 3.36 mm) but a decrease in the thickness of 0.13 mm in the SVG wall thickness over this time.[12] The mechanism underlying this negative remodeling thought to be the reaction of the venous conduit to the greater than normal flow, wall stretch, shear stress as well as humoral factors such as cytokines and vasoactive substances.
Late failure of saphenous vein grafts
Multiple pathophysiologic processes contribute to late graft degeneration or late graft failure. These processes including intimal hyperplasia, atherosclerotic plaque formation, and graft remodeling. Additionally, arterialization of the graft accelerates atherosclerosis. In addition to mechanically obstructing flow, these blockages are more "friable" (i.e. they easily break into small pieces and embolize downstream into the myocardium impairing perfusion) and more prone to thrombus than plaques found in native vessels. Another pathophysiologic mechanism whereby SVGs are more susceptible to thrombotic occlusion is the fact that they lack side branches.
Saphenous vein graft patency
Definitions
The rate of saphenous vein graft failure varies depending upon the definition used and the nature of the study design. [13][14][15][16] Rates estimated based upon retrospective studies of patients who are symptomatic underestimate the true rate of SVG failure because only those patients who survive who are symptomatic undergo catheterization. The most accurate assessment is based upon prospective studies in which all patients undergo mandatory cardiac catheterization at a uniform timepoint. The rates will also vary depending upon the complexity of disease, the diffuse nature of the disease, the extent of revascularization, and whether a per-lesion or a per-patient analysis is undertaken.
Saphenous vein graft occlusion is defined as a complete, 100% occlusion of a saphenous vein graft. [17]
Saphenous vein failure is defined as an occlusion of the vein graft or a 75% or greater stenosis.
The rate of occlusion or failure of saphenous vein grafts is calculated on a per graft basis and a per patient basis. The per patient basis is higher, because only one vein graft out of several must fail for the patient to be characterized as a failure.
Current rates of graft occlusion and failure are as follows:[17]
The rate of per patient vein graft occlusion at 12-18 months is about 42%
The rate of per patient vein graft failure at 12-18 months is about 46%
The rate of per graft vein graft occlusion at 12-18 months is about 26%
The rate of per graft vein graft failure at 12-18 months is about 29%
As a comparison, the rate of internal mammary artery failure at 12-18 months was only 8%.
Time course of SVG failure
- Early failure: Withing the first month, 8% to 18% of SVGs fail again largely due to the factors cited above that precipitate thrombosis. [18][19]
- Mid course failure: From one month through a year an additional 10—15% of SVGs occlude. Again, during this period the pathophysiology is predominantly due to smooth muscle cell hyperplasia emanating from the intima of the vein.[8]
- Late Failure:After year 1, the annual rate of occlusion is about 1—4% per per year. Again, this process of late failure is predominantly due to atherosclerosis and some intimal hyperplasia.
- Very Late Failure: SVG occlusion after 5 years is predominantly mediated by atherosclerosis. A convenient and often quoted statistic is that at 10 years, approximately 50% of SVGs remain patent[20]
Determinants of sapheous vein graft patency
Graft patency is dependent on a number of factors, including the type of graft used (internal thoracic artery, radial artery, or great saphenous vein), the size or the coronary artery that the graft is anastomosed with, and, of course, the skill of the surgeon(s) performing the procedure. Arterial grafts (e.g. left internal mammary (LIMA), radial) are far more sensitive to rough handling than the saphenous veins and may go into spasm if handled improperly.
Technical factors during surgery
A large VA cooperative study evaluated the technical factors associated with 3 year patency among those SVGs that were patent at 7 to 10 days following CABG. SVG occlusion was associated with the following:[21]
- Cross-clamp time > 80 min (p < 0.001)
- Vein preservation solution temperature > 5 degrees C (p = 0.009)
- Bypass time > 2 hours (p = 0.042)
- Number of proximal anastomoses > 2 (p = 0.018)
- Operation time > 5 hours (p = 0.044)
- Intermittent instead of continuous cross-clamp technique (p = 0.024).
- Sequential vs single Y vein graft (p = 0.060).
In-situ vs free grafts
Generally the best patency rates are achieved with the in-situ (the proximal end is left connected to the subclavian artery) left internal thoracic artery (a LIMA) with the distal end being anastomosed with the coronary artery (typically the left anterior descending artery or a diagonal branch artery). Lesser patency rates can be expected with radial artery grafts and "free" internal thoracic artery grafts (where the proximal end of the thoracic artery is excised from its origin from the subclavian artery and re-anastomosed with the ascending aorta).
Venous vs Arterial conduits
Saphenous vein grafts have poorer patency rates than arterial grafts, but are more available, as the patients can have multiple segments of the saphenous vein used to bypass different arteries.
LITA grafts are longer-lasting than vein grafts, both because the artery is more robust than a vein and because, being already connected to the arterial tree, the LITA need only be grafted at one end. The LITA is usually grafted to the left anterior descending coronary artery (LAD) because of its superior long-term patency when compared to saphenous vein grafts.[22][23]
Impact of harvesting method on saphenous vein graft patency
The method of harvesting vein grafts may be associated with late vein graft patency. In one small study of 40 patients randomized to endoscopic vs traditional techniques, no difference was seen in angiographic patency at 3 months.[6]Another small randomized study of 144 patients who returned for angiography at 6 months and demonstrated an occlusion rate of 21.7% for the endoscopic approach vs 17.6% for the open approach.[5] In a non-randomized subgroup analysis from the PREVENT IV study, harvesting of vein-grafts with the use of endoscopy (endoscopic harvesting) was associated with a higher rate of saphenous vein graft failure at 12-18 months compared with open harvesting of the veins under direct visualization (46.7% vs. 38.0%, P<0.001 at 12-18 months).[4] Likewise, clinical outcomes were worse at 3 years: use of endoscopy was associated with higher rates of death, myocardial infarction, or repeat revascularization (20.2% vs. 17.4%; p=0.04), death or myocardial infarction (9.3% vs. 7.6%; p=0.01), and death (7.4% vs. 5.8%; adjusted hazard ratio, 1.52; 95% CI, 1.13 to 2.04; p=0.005). Although these observational data are provocative, further randomized clinical trials involving large numbers of patients from multiple centers with long term follow-up would be needed to compare the safety and effectiveness of the two harvesting techniques.
Perioperative MI is associated with a higher rate of SVG failure
The rate of one-year saphenous vein graft failure has been documented to be 62.4% of patients with and 43.8% of patients without perioperative MI (p <0.001).[24]
Smaller target vessels are associated with a higher rate of SVG failure
The rate of SVG occlusion at one year is about twice as high in those SVGs that are anastomosed to a target vessel with a diameter < 2.0 mm.[25] The rate of SVG occlusion at one year in target vessels less than or equal to 2.0 mm in diameter was 20.1% on aspirin and 32.3% off aspirin (p = 0.008), while in those SVGs anastomosed to target vessels > 2.0 mm in diameter the rates were lower: 8.7% and 9.0% respectively. The converse of this, is that larger conduits have been associated with higher rates of SVG occlusion.[5]
Target artery location
In a multivariate model in a small study, SVG grafting to the diagonal branch has been associated with 1.76 times higher rates of SVG occlusion.[5]
Graft flow
Poor graft flow has been associated with higher rates of SVG occlusion.[5]
Serum cholesterol
A serum cholesterol > 225 mg/dl has been associated with higher rates of SVG failure at 3 years in a multivariate model from a large VA cooperative study.[21]
Pharmacotherapy
Early post-operative aspirin has been associated with a lower rate of SVG failure for the first 3 years after CABG in a large number of randomized trials.[26][27][28][29][30][31][32][33] [34][35][36][37][38][39]
Factors not associated with saphenous vein graft patency
Although a creatinine clearance < 60 ml/sec has been associated with higher rates of death, MI, and revascularization, it was not associated with a higher rate of SVG or internal thoracic artery failure rates. [40] In one large VA cooperative study, age, race, smoking history, high density lipoprotein cholesterol, vein source (thigh vs. calf) were not associated with 3 year SVG patency.[21]
Association of saphenous vein graft failure with clinical events
In the PREVENT IV study, SVG failure was associated with a 13.9% rate of death and MI (122/878) vs 0.9% (9/1,042) for those patients without SVG failure (these numbers exclude peri-operative MI).[17] Likewise, the rate of death, MI, and revascularization was higher among patients with SVG failure (26.0% vs 1.8%). Despite these elevated rates of adverse events, it shoud be noted that about half of the patients with SVG failure did not have clinical events. This may be because the native artery remained open or because there was extensive collaterals. It should slo be noted that the development of heart failure or angina following SVG failure may not be captured in the endpoint of death, MI, and revascularization.
In a large cohort of 1,388 patients who underwent a first coronary artery bypass graft procedure, vein graft patency was temporally related both to reoperation as well as survival.[41]
Other non-atherosclerotic saphenous vein graft diseases
Saphenous vein graft aneurysms
This disease process is also known as SVGA, aortocoronary saphenous vein graft aneurysms, saphenous vein graft aneurysm disease, or saphenous vein graft aneurysmal dilatation and is defined as a local dilation of the vessel more than 1.5X the adjacent reference segment. The aneurysms can be up to 14 cm in diameter.
Classification
- True aneurysms: All 3 layers of the vessel wall are involved
- Pseudoaneurysms: There is disruption of 1 or more layers of the vessel wall.
Epidemiology and demographics
Over the course f a SVGs 7 year lifetime, the risk of aneurysm development is 14%. True aneurysms outnumber false ones by a ratio of 2:1.
Pathophysiology
Causes of saphenous vein graft aneurysms include the following:
- Atherosclerosis
- Hypertension
- Mycotic
- Postoperative mediastinitis
- Previous aneurysms
- Torn sutures
Natural history and complications
SVGAs can rupture which is associated with a high rate of morbidity and even mortality. They can also be a nidus for embolization.
Diagnosis
History
If a patient with a history of CABG develops chest pain and has a mediastinal mass, an SVGA should be suspected.
The majority of patients are asymptomatic with a true aneurysm, and most often the SVGA is an incidental finding on an imaging study. If the patient is symptomatic, about half the time it presents as an acute coronary syndrome. Very rarely tamponade from compression of the right atrium or ischemia due to compression of the left internal mammary artery bypass graft has been observed.
In contrast to true aneurysms, patients with false aneurysms are symptomatic in 85% of cases. About two thirds of the time they present with an acute coronary syndrome. If a patient with an SVGA does present with chest pain or hemoptysis, it may be due to the formation of a fistula.
Physical examination
Rarely on physical examination a murmur will be auscultated or cutaneous bleeding will be observed (both due to a fistula).
Imaging
SVGA can be definitively diagnosed on either coronary angiography or CT angiography. On occasion, an SVGA can be observed as either hilar or mediastinal mass on chest x ray.
Management
Pharmacologic management consists of aspirin and lipid-lowering therapy. The benefit if any of coumadin and beta-blockers is not known.
A surgery or a percutaneous intervention is suggested if:
- A pseudoaneurysm is present
- The aneurysm is more than 2 cm greater than the adjacent vessel
- A fistula is present (surgery, coiling, or stenting)
- If the aneurysm is mycotic (surgery)
Surgery
There are multiple surgical approaches to repairing an aneurysm:
- Ligate the aneurysm-containing SVG and place a new SVG.
- Resect the aneurysmal portion of the diseased graft and sew a new SVG segment in in an end-to-end fashion
- Ligate the old SVG without revascularization
- Evacuate the hematoma and repair the SVG with a venous patch graft.
Percutaneous intervention
In the past, percutaneous intervention was reserved for patients who were too sick to undergo surgery. However, due to the improved tools that are available, more patients are undergoing percutaneous intervention as described below:
- Coil embolization: This technique has evolved so that a stent excludes the coil form lying in the lumen of the SVG.
- Covered stents: The JOSTENT Coronary Stent Graft (Abbott Vascular, Redwood City, Calif) can be used to exclude the aneurysm form the body of the SVG. The device is made up of an ultra-thin layer of polytetrafluoroethylene (PTFE).
- Multiple overlapping stents can be used to exclude the aneurysm.
Amyloidosis of saphenous coronary bypass grafts
Amyloid has been associated with accelarated disease in saphenous vein grafts.[42] [43] [44] [45] [46]
Rupture of the saphenous vein coronary artery bypass grafts
Aspergillus species causing a necrotizing vasculitis have been associated with rupture of a saphenous vein grafts.
References
- ↑ Lawrie GM, Weilbacher DE, Henry PD. Endothelium-dependent relaxation in human saphenous vein grafts. Effects of preparation and clinicopathologic correlations. J Thorac Cardiovasc Surg 1990;100:612—20.
- ↑ Souza DS, Johansson B, Bojo¨ L, Karlsson R, Geijer H, Filbey D, Bodin L, Arbeus M, Dashwood MR. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 2006;132:373—8.
- ↑ 3.0 3.1 Kiaii B, Moon BC, Massel D, Langlois Y, Austin TW, Willoughby A, Guiraudon C, Howard CR, Guo LR (2002). "A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery". J. Thorac. Cardiovasc. Surg. 123 (2): 204–12. PMID 11828277. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 4.2 Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, Mehta RH, Gibson CM, Mack MJ, Kouchoukos NT, Califf RM, Alexander JH (2009). "Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery". The New England Journal of Medicine. 361 (3): 235–44. doi:10.1056/NEJMoa0900708. PMID 19605828. Retrieved 2010-07-12. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Yun KL, Wu Y, Aharonian V, Mansukhani P, Pfeffer TA, Sintek CF, Kochamba GS, Grunkemeier G, Khonsari S (2005). "Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: six-month patency rates". J. Thorac. Cardiovasc. Surg. 129 (3): 496–503. doi:10.1016/j.jtcvs.2004.08.054. PMID 15746730. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 6.2 Perrault LP, Jeanmart H, Bilodeau L, Lespérance J, Tanguay JF, Bouchard D, Pagé P, Carrier M (2004). "Early quantitative coronary angiography of saphenous vein grafts for coronary artery bypass grafting harvested by means of open versus endoscopic saphenectomy: a prospective randomized trial". J. Thorac. Cardiovasc. Surg. 127 (5): 1402–7. doi:10.1016/j.jtcvs.2003.10.040. PMID 15115999. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ Ohki S, Kaneko T, Satoh Y; et al. (2002). "[Coronary artery bypass grafting in octogenarian]". Kyobu geka. The Japanese journal of thoracic surgery (in Japanese). 55 (10): 829–33, discussion 833–6. PMID 12233100.
- ↑ 8.0 8.1 Motwani JG, Topol EJ (1998). "Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention". Circulation. 97 (9): 916–31. PMID 9521341. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Lau GT, Lowe HC, Kritharides L (2004). "Cardiac saphenous vein bypass graft disease". Semin Vasc Med. 4 (2): 153–9. doi:10.1055/s-2004-835373. PMID 15478036. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Yamada T, Itoh T, Nakano S, Tokunaga O (1995). "Time-dependent thickening of the intima in aortocoronary saphenous vein grafts: clinicopathological analysis of 24 patients". Heart Vessels. 10 (1): 41–5. PMID 7730246.
|access-date=requires|url=(help) - ↑ Shi Y, Pieniek M, Fard A, O'Brien J, Mannion JD, Zalewski A (1996). "Adventitial remodeling after coronary arterial injury". Circulation. 93 (2): 340–8. PMID 8548908. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, Lowe HC, Freedman BS, Kritharides L (2006). "Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness". Circulation. 114 (1 Suppl): I435–40. doi:10.1161/CIRCULATIONAHA.105.001008. PMID 16820615. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ FitzGibbon GM, Leach AJ, Keon WJ, Burton JR, Kafka HP. Coronary bypass graft fate. J Thorac Cardiovasc Surg. 1986;91:773-778.
- ↑ Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome. J AmColl Cardiol. 1996;28:616-626.
- ↑ Desai ND, Cohen EA, Naylor CD, Fremes SE; Radial Artery Patency Study Investigators. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl JMed. 2004;351:2302-2309.
- ↑ Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery. J Am Coll Cardiol. 2004;44:2149-2156.
- ↑ 17.0 17.1 17.2 Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT (2005). "Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial". JAMA : the Journal of the American Medical Association. 294 (19): 2446–54. doi:10.1001/jama.294.19.2446. PMID 16287955. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Fuster V, Chesebro JH. Aorto-coronary artery vein graft disease: experimental and clinical approach for the understanding of the role of platelets and platelet inhibitors. Circulation 1985;72(Suppl. V):65—70.
- ↑ Fuster V, Chesebro JH. Role of platelets and platelet inhibitors in aortocoronary artery vein-graft disease. Circulation 1986;73:227—32.
- ↑ Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616—26.
- ↑ 21.0 21.1 21.2 Goldman S, Zadina K, Krasnicka B, Moritz T, Sethi G, Copeland J, Ovitt T, Henderson W (1997). "Predictors of graft patency 3 years after coronary artery bypass graft surgery. Department of Veterans Affairs Cooperative Study Group No. 297". J. Am. Coll. Cardiol. 29 (7): 1563–8. PMID 9180120. Retrieved 2010-07-23. Unknown parameter
|month=ignored (help) - ↑ Kitamura S, Kawachi K, Kawata T, Kobayashi S, Mizuguchi K, Kameda Y, Nishioka H, Hamada Y, Yoshida Y. [Ten-year survival and cardiac event-free rates in Japanese patients with the left anterior descending artery revascularized with internal thoracic artery or saphenous vein graft: a comparative study] Nippon Geka Gakkai Zasshi. 1996 Mar;97(3):202-9. PMID 8649330.
- ↑ Arima M, Kanoh T, Suzuki T, Kuremoto K, Tanimoto K, Oigawa T, Matsuda S. Serial Angiographic Follow-up Beyond 10 Years After Coronary Artery Bypass Grafting. Circ J. 2005 Aug;69(8):896-902. PMID 16041156. [1].
- ↑ Yau JM, Alexander JH, Hafley G, Mahaffey KW, Mack MJ, Kouchoukos N, Goyal A, Peterson ED, Gibson CM, Califf RM, Harrington RA, Ferguson TB (2008). "Impact of perioperative myocardial infarction on angiographic and clinical outcomes following coronary artery bypass grafting (from PRoject of Ex-vivo Vein graft ENgineering via Transfection [PREVENT] IV)". The American Journal of Cardiology. 102 (5): 546–51. doi:10.1016/j.amjcard.2008.04.069. PMID 18721510. Retrieved 2010-07-14. Unknown parameter
|month=ignored (help) - ↑
ref name="pmid2680158">Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y (1989). "Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study". Circulation. 80 (5): 1190–7. PMID 2680158. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y (1988). "Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study". Circulation. 77 (6): 1324–32. PMID 3286040. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y (1989). "Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration Cooperative Study". Circulation. 80 (5): 1190–7. PMID 2680158. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S (1994). "Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: results of a VA Cooperative study". Circulation. 89 (3): 1138–43. PMID 8124800. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Chesebro JH, Clements IP, Fuster V, Elveback LR, Smith HC, Bardsley WT, Frye RL, Holmes DR, Vlietstra RE, Pluth JR, Wallace RB, Puga FJ, Orszulak TA, Piehler JM, Schaff HV, Danielson GK (1982). "A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency". N. Engl. J. Med. 307 (2): 73–8. PMID 7045659. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Chesebro JH, Fuster V, Elveback LR, Clements IP, Smith HC, Holmes DR, Bardsley WT, Pluth JR, Wallace RB, Puga FJ (1984). "Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations". N. Engl. J. Med. 310 (4): 209–14. PMID 6361561. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Mangano DT (2002). "Aspirin and mortality from coronary bypass surgery". N. Engl. J. Med. 347 (17): 1309–17. doi:10.1056/NEJMoa020798. PMID 12397188. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Lorenz RL, Schacky CV, Weber M; et al. (1984). "Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily). Effects on platelet aggregation and thromboxane formation". Lancet. 1 (8389): 1261–4. PMID 6144975. Unknown parameter
|month=ignored (help) - ↑ Hockings BE, Ireland MA, Gotch-Martin KF, Taylor RR (1993). "Placebo-controlled trial of enteric coated aspirin in coronary bypass graft patients. Effect on graft patency". Med. J. Aust. 159 (6): 376–8. PMID 8377686. Unknown parameter
|month=ignored (help) - ↑ Sanz G, Pajarón A, Alegría E; et al. (1990). "Prevention of early aortocoronary bypass occlusion by low-dose aspirin and dipyridamole. Grupo Español para el Seguimiento del Injerto Coronario (GESIC)". Circulation. 82 (3): 765–73. PMID 2203555. Unknown parameter
|month=ignored (help) - ↑ Gavaghan TP, Gebski V, Baron DW (1991). "Immediate postoperative aspirin improves vein graft patency early and late after coronary artery bypass graft surgery. A placebo-controlled, randomized study". Circulation. 83 (5): 1526–33. PMID 2022014. Unknown parameter
|month=ignored (help) - ↑ Sharma GV, Khuri SF, Josa M, Folland ED, Parisi AF (1983). "The effect of antiplatelet therapy on saphenous vein coronary artery bypass graft patency". Circulation. 68 (3 Pt 2): II218–21. PMID 6347428. Unknown parameter
|month=ignored (help) - ↑ Brown BG, Cukingnan RA, DeRouen T; et al. (1985). "Improved graft patency in patients treated with platelet-inhibiting therapy after coronary bypass surgery". Circulation. 72 (1): 138–46. PMID 3874009. Unknown parameter
|month=ignored (help) - ↑ McEnany MT, Salzman EW, Mundth ED; et al. (1982). "The effect of antithrombotic therapy on patency rates of saphenous vein coronary artery bypass grafts". J. Thorac. Cardiovasc. Surg. 83 (1): 81–9. PMID 7033673. Unknown parameter
|month=ignored (help) - ↑ Goldman S, Copeland J, Moritz T; et al. (1990). "Internal mammary artery and saphenous vein graft patency. Effects of aspirin". Circulation. 82 (5 Suppl): IV237–42. PMID 2225410. Unknown parameter
|month=ignored (help) - ↑ Mehta RH, Hafley GE, Gibson CM, Harrington RA, Peterson ED, Mack MJ, Kouchoukos NT, Califf RM, Ferguson TB, Alexander JH (2008). "Influence of preoperative renal dysfunction on one-year bypass graft patency and two-year outcomes in patients undergoing coronary artery bypass surgery". The Journal of Thoracic and Cardiovascular Surgery. 136 (5): 1149–55. doi:10.1016/j.jtcvs.2008.02.085. PMID 19026795. Retrieved 2010-07-14. Unknown parameter
|month=ignored (help) - ↑ Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR (1996). "Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years". J. Am. Coll. Cardiol. 28 (3): 616–26. PMID 8772748. Retrieved 2010-07-22. Unknown parameter
|month=ignored (help) - ↑ Marti MC, Bouchardy B, Cox JN. Aortocoronary bypass with autogenous saphenous vein grafts: histopathological aspects. Virchows Arch Abt A Path Anat 1971; 352: 255–66.
- ↑ Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft. JAMA 1973; 223: 792–4.
- ↑ Zemva A, Ferluga D, Zorc M, Popovic M, Porenta OV, Radovanovic N. Amyloidosis in saphenous vein aortocoronary bypass grafts. J Cardiovasc Surg 1990; 31: 441–4.
- ↑ Salerno TA, Wasan SM, Charrette EJ. Prospective analysis of heart biopsies in coronary artery surgery. Ann Thorac Surg 1979; 28: 436–9.
- ↑ Pelosi F, Capehart J, Roberts WC. Effectiveness of cardiac transplantation for primary (AL) cardiac amyloidosis. Am J Cardiol 1997; 79: 532–5.