Conivaptan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 77: | Line 77: | ||
|useInNursing=It is not known whether conivaptan is excreted in human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from VAPRISOL, a decision should be made to discontinue nursing or VAPRISOL, taking into consideration the importance of VAPRISOL to the mother. Conivaptan is excreted in milk and detected in neonates when given by intravenous administration to lactating rats. Milk levels of conivaptan in rats reached maximal levels at 1 hour post dose following intravenous administration and were up to 3 times greater than maternal plasma levels following an intravenous dose of 1 mg/kg (systemic exposure less than human therapeutic exposure based on AUC comparison). | |useInNursing=It is not known whether conivaptan is excreted in human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from VAPRISOL, a decision should be made to discontinue nursing or VAPRISOL, taking into consideration the importance of VAPRISOL to the mother. Conivaptan is excreted in milk and detected in neonates when given by intravenous administration to lactating rats. Milk levels of conivaptan in rats reached maximal levels at 1 hour post dose following intravenous administration and were up to 3 times greater than maternal plasma levels following an intravenous dose of 1 mg/kg (systemic exposure less than human therapeutic exposure based on AUC comparison). | ||
|useInPed=The safety and effectiveness of VAPRISOL in pediatric patients have not been studied. | |useInPed=The safety and effectiveness of VAPRISOL in pediatric patients have not been studied. | ||
|useInGeri=In clinical studies of VAPRISOL administered as a 20 mg IV loading dose followed by 20 mg/day or 40 mg/day IV for 2 to 4 days, 89% (20 mg/day regimen) and 60% (40 mg/day regimen) of participants were greater than or equal to 65 years of age and 60% (20 mg/day regimen) and 40% (40 mg/day regimen) were greater than or equal to 75 years of age. In general, the adverse event profile in elderly patients was similar to that seen in the general study population. | |useInGeri=In clinical studies of VAPRISOL administered as a 20 mg IV loading dose followed by 20 mg/day or 40 mg/day IV for 2 to 4 days, 89% (20 mg/day regimen) and 60% (40 mg/day regimen) of participants were greater than or equal to 65 years of age and 60% (20 mg/day regimen) and 40% (40 mg/day regimen) were greater than or equal to 75 years of age. In general, the adverse event profile in elderly patients was similar to that seen in the general study population. | ||
|useInRenalImpair=No clinically relevant increase in exposure was observed in subjects with mild and moderate renal impairment (CLcr 30 – 80 mL/min). No dose adjustment of VAPRISOL is necessary. Because of the high incidence of infusion site phlebitis (which can reduce vascular access sites) and unlikely benefit, use in patients with severe renal impairment (CLcr <30 mL/min) is not recommended [see Clinical Pharmacology (12.3)]. | |useInRenalImpair=No clinically relevant increase in exposure was observed in subjects with mild and moderate renal impairment (CLcr 30 – 80 mL/min). No dose adjustment of VAPRISOL is necessary. Because of the high incidence of infusion site phlebitis (which can reduce vascular access sites) and unlikely benefit, use in patients with severe renal impairment (CLcr <30 mL/min) is not recommended [see Clinical Pharmacology (12.3)]. | ||
|useInHepaticImpair=No clinically relevant increase in exposure was observed in subjects with mild hepatic impairment; therefore no dose adjustment of VAPRISOL is necessary. The exposure to VAPRISOL approximately doubles with moderate hepatic impairment. The impact of severe hepatic impairment on the exposure to conivaptan has not been studied [see Dosage and Administration (2.3) andClinical Pharmacology(12.3)]. | |useInHepaticImpair=No clinically relevant increase in exposure was observed in subjects with mild hepatic impairment; therefore no dose adjustment of VAPRISOL is necessary. The exposure to VAPRISOL approximately doubles with moderate hepatic impairment. The impact of severe hepatic impairment on the exposure to conivaptan has not been studied [see Dosage and Administration (2.3) andClinical Pharmacology(12.3)]. | ||
|administration====== General Dosing Information ===== | |||
VAPRISOL is for intravenous use only.<BR> | |||
VAPRISOL is for use in hospitalized patients only.<BR> | |||
Administer VAPRISOL through large veins and change of the infusion site every 24 hours to minimize the risk of vascular irritation [seeWarnings and Precautions (5.5)].<BR> | |||
Initiate with a loading dose of 20 mg IV administered over 30 minutes.<BR> | |||
Follow the loading dose with 20 mg of VAPRISOL administered in a continuous intravenous infusion over 24 hours. After the initial day of treatment, administer VAPRISOL for an additional 1 to 3 days in a continuous infusion of 20 mg/day. If serum sodium is not rising at the desired rate, VAPRISOL may be titrated upward to a dose of 40 mg daily, administered in a continuous intravenous infusion.<BR> | |||
The total duration of infusion of VAPRISOL (after the loading dose) should not exceed four days. The maximum daily dose of VAPRISOL (after the loading dose) is 40 mg/day.<BR> | |||
Patients receiving VAPRISOL must have frequent monitoring of serum sodium and volume status. An overly rapid rise in serum sodium (> 12 mEq/L/24 hours) may result in serious neurologic sequelae. For patients who develop an undesirably rapid rate of rise of serum sodium, VAPRISOL should be discontinued, and serum sodium and neurologic status should be carefully monitored. If the serum sodium continues to rise, VAPRISOL should not be resumed. If hyponatremia persists or recurs, and the patient has had no evidence of neurologic sequelae of rapid rise in serum sodium, VAPRISOL may be resumed at a reduced dose [see Warnings and Precautions (5.2)]. For patients who develop hypovolemia or hypotension while receiving VAPRISOL, VAPRISOL should be discontinued, and volume status and vital signs should be frequently monitored. Once the patient is again euvolemic and is no longer hypotensive, VAPRISOL may be resumed at a reduced dose if the patient remains hyponatremic.<BR> | |||
===== Preparation, Compatibility and Stability ===== | |||
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter, discoloration or cloudiness is observed, the drug solution should not be used.<BR> | |||
VAPRISOL is supplied in a single-use 100 mL flexible INTRAVIA Container containing a sterile premixed dilute, ready-to-use, nonpyrogenic solution of conivaptan hydrochloride, 0.2 mg per mL (20 mg/100 mL) in 5% Dextrose. NO FURTHER DILUTION OF THIS PREPARATION IS NECESSARY. | |||
VAPRISOL is compatible with 5% Dextrose Injection. VAPRISOL is physically and chemically compatible with 0.9% Sodium Chloride Injection for up to 48 hours when the two solutions are co-administered via a Y-site connection at a flow rate for VAPRISOL of 4.2 mL/hour and at flow rates for 0.9% Sodium Chloride Injection of either 2.1 mL/hour or 6.3 mL/hour. <BR> | |||
VAPRISOL has been shown to be incompatible with both Lactated Ringer's Injection and furosemide injection when these products are mixed in the same container; therefore, do not combine VAPRISOL with these products in the same intravenous line or container.<BR> | |||
VAPRISOL should also not be combined with any other product in the same intravenous line or container.<BR> | |||
<u>Loading Dose</u> | |||
Administer the content of a 20 mg/100 mL VAPRISOL flexible plastic container over 30 minutes. | |||
<u>Continuous Infusion</u> | |||
For patients requiring 20 mg VAPRISOL injection per day, administer the content of one 20 mg/100 mL VAPRISOL flexible plastic container over 24 hours.<BR> | |||
For patients requiring 40 mg VAPRISOL injection per day, administer the content of two consecutive 20 mg/100 mL VAPRISOL flexible plastic containers over 24 hours.<BR> | |||
'''Since the flexible container is for single-use only, any unused portion should be discarded.'''<BR> | |||
'''CAUTION: Do not use plastic containers in series connections.''' Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.<BR> | |||
Do not remove container from overwrap until ready for use. The overwrap is a moisture and light barrier. The inner container maintains the sterility of the product.<BR> | |||
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. After removing overwrap, check for minute leaks by squeezing inner container firmly. If leaks are found, discard solution as sterility may be impaired. Do not use if the solution is cloudy or a precipitate is present.<BR> | |||
DO NOT ADD SUPPLEMENTARY MEDICATION. | |||
Preparation for Administration: | |||
* Suspend container from eyelet support. | |||
* Remove protector from outlet port at bottom of container. | |||
* Attach administration set. Refer to complete directions accompanying set. | |||
===== Hepatic Impairment ===== | |||
In patients with moderate hepatic impairment, initiate VAPRISOL with a loading dose of 10 mg over 30 minutes followed by 10 mg per day as a continuous infusion for 2 to 4 days. If serum sodium is not rising at the desired rate, VAPRISOL may be titrated upward to 20 mg per day [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. | |||
}} | }} | ||
Revision as of 15:42, 22 April 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Conivaptan is a Vasopressin antagonist that is FDA approved for the {{{indicationType}}} of raising serum sodium in hospitalized patients with euvolemic and hypervolemic hyponatremia.. Common adverse reactions include hypertension, orthostatic hypotension, peripheral edema, phlebitis, injection site reaction, hypokalemia, increased thirst, constipation, diarrhea, vomiting, headache, increased frequency of urination, polyuria, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
<h4>Condition 1</h4>
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Conivaptan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Hypovolemic Hyponatremia
- VAPRISOL is contraindicated in patients with hypovolemic hyponatremia.
- Coadministration with Potent CYP3A Inhibitors
- The coadministration of VAPRISOL with potent CYP3A inhibitors, such as ketoconazole, itraconazole, clarithromycin, ritonavir, and indinavir, is contraindicated [see Drug Interactions (7.1)].
- Anuric Patients
- In patients unable to make urine, no benefit can be expected [see Clinical Pharmacology (12.3)].
- Known Allergy to Corn or Corn Products
- Solutions containing dextrose are contraindicated in patients with known allergy to corn or corn products.
Warnings
- Hypovolemic hyponatremia
- VAPRISOL is contraindicated in patients with hypovolemic hyponatremia.
- Coadministration with Potent CYP3A Inhibitors
- The coadministration of VAPRISOL with potent CYP3A inhibitors, such as ketoconazole, itraconazole, clarithromycin, ritonavir, and indinavir, is contraindicated [see Drug Interactions (7.1)].
- Anuric Patients
- In patients unable to make urine, no benefit can be expected [see Clinical Pharmacology (12.3)].
- Known Allergy to Corn or Corn Products
- Solutions containing dextrose are contraindicated in patients with known allergy to corn or corn products.
- hyponatremia Associated with Heart Failure
- The amount of safety data on the use of VAPRISOL in patients with hypervolemic hyponatremia associated with heart failure is limited. VAPRISOL should be used to raise serum sodium in such patients only after consideration of other treatment options [see Adverse Reactions (6.1)].
- Overly Rapid Correction of Serum Sodium
- Osmotic demyelination syndrome is a risk associated with overly rapid correction of hyponatremia (i.e., > 12 mEq/L/24 hours). Osmotic demyelination results in dysarthria, mutism, dysphagia, lethargy, affective changes, spastic quadriparesis, seizures, coma or death. In susceptible patients, including those with severe malnutrition, alcoholism or advanced liver disease, use slower rates of correction. In controlled clinical trials of VAPRISOL, about 9% of patients who received VAPRISOL in doses of 20-40 mg/day IV had rises of serum sodium >12 mEq/L/24 hours, but none of these patients had evidence of osmotic demyelination or permanent neurologic sequelae. Serum sodium concentration and neurologic status should be monitored appropriately during VAPRISOL administration, and VAPRISOL administration should be discontinued if the patient develops an undesirably rapid rate of rise of serum sodium. If the serum sodium concentration continues to rise, VAPRISOL should not be resumed. If hyponatremia persists or recurs (after initial discontinuation of VAPRISOL for an undesirably rapid rate of rise of serum sodium concentration), and the patient has had no evidence of neurologic sequelae of rapid rise in serum sodium, VAPRISOL may be resumed at a reduced dose [see Dosage and Administration (2.1)].
- Coadministration of VAPRISOL and Drugs Eliminated Primarily by CYP3A Mediated Metabolism
- In clinical trials of oral conivaptan, two cases of rhabdomyolysis occurred in patients who were also receiving a CYP3A-metabolized HMG-CoA reductase inhibitor. Avoid concomitant use of VAPRISOL with drugs eliminated primarily by CYP3A-mediated metabolism. Subsequent treatment with CYP3A substrate drugs may be initiated no sooner than 1 week after the infusion of VAPRISOL is completed [see Drug Interactions (7.1)].
- Coadministration of VAPRISOL and digoxin
- Infusion Site Reactions
- Infusion site reactions are common and can include serious reactions, even with proper infusion rates [see Adverse Reactions (6.1)]. Administer VAPRISOL via large veins, and rotate the infusion site every 24 hours [see Dosage and Administration (2.1)].
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse event information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates. The most common adverse reactions reported with VAPRISOL administration were infusion site reactions. In studies in patients and healthy volunteers, infusion site reactions occurred in 73% and 63% of subjects treated with VAPRISOL 20 mg/day and 40 mg/day, respectively, compared to 4% in the placebo group. Infusion site reactions were the most common type of adverse event leading to discontinuation of VAPRISOL. Discontinuations from treatment due to infusion site reactions were more common among VAPRISOL-treated patients (3%) than among placebo-treated patients (0%). Some serious infusion site reactions did occur [see Dosage and Administration (2.1) and Warnings and Precautions (5.5)]. The adverse reactions presented in Table 1 are derived from 72 healthy volunteers and 243 patients with euvolemic or hypervolemic hyponatremia who received VAPRISOL 20 mg IV as a loading dose followed by 40 mg/day IV for 2 to 4 days, from 37 patients with euvolemic or hypervolemic hyponatremia who received VAPRISOL 20 mg IV as a loading dose followed by 20 mg/day IV for 2 to 4 days in an open-label study, and from 40 healthy volunteers and 29 patients with euvolemic or hypervolemic hyponatremia who received placebo. The adverse reactions occurred in at least 5% of patients treated with VAPRISOL and at a higher incidence for VAPRISOL-treated patients than for placebo-treated patients.
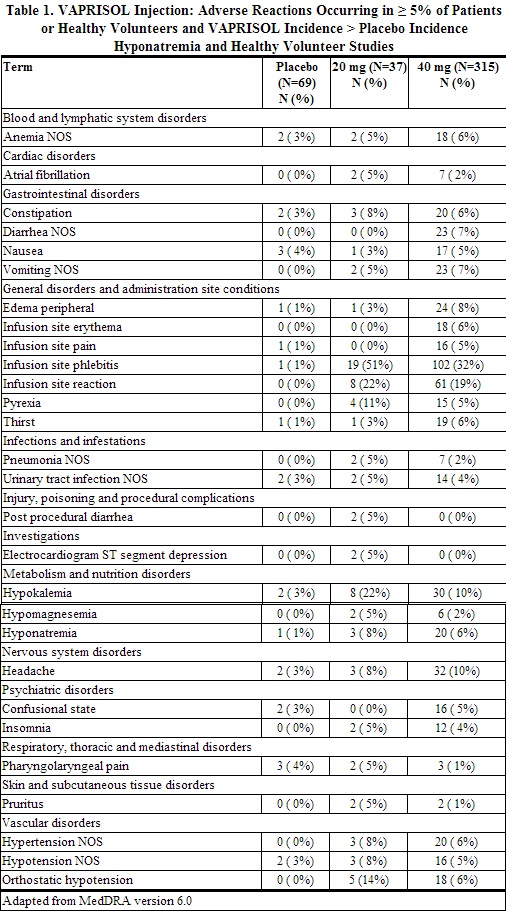
Although a dose of 80 mg/day of VAPRISOL was also studied, it was associated with a higher incidence of infusion site reactions and a higher rate of discontinuation for adverse events than was the 40 mg/day VAPRISOL dose. The maximum recommended daily dose of VAPRISOL (after the loading dose) is 40 mg/day.
Heart failure with hypervolemic hyponatremia
In clinical trials where VAPRISOL was administered to 79 hypervolemic hyponatremic patients with underlying heart failure and intravenous placebo administered to 10 patients, adverse cardiac failure events, atrial dysrhythmias, and sepsis occurred more frequently among patients treated with VAPRISOL (32%, 5% and 8% respectively) than among patients treated with placebo (20%, 0% and 0% respectively) [see Warnings and Precautions (5.1)].
Postmarketing Experience
There is limited information regarding Conivaptan Postmarketing Experience in the drug label.
Drug Interactions
- CYP3A
- Conivaptan is a sensitive substrate of CYP3A. The effect of ketoconazole, a potent CYP3A inhibitor, on the pharmacokinetics of intravenous conivaptan has not been evaluated. Coadministration of oral conivaptan hydrochloride 10 mg with ketoconazole 200 mg resulted in 4- and 11-fold increases in Cmax and AUC of conivaptan, respectively [see Contraindications (4.2)].
- Conivaptan is a potent mechanism-based inhibitor of CYP3A. The effect of conivaptan on the pharmacokinetics of co-administered CYP3A substrates has been evaluated with the coadministration of conivaptan with midazolam, simvastatin, and amlodipine. VAPRISOL 40 mg/day increased the mean AUC values by approximately 2- and 3-fold for 1 mg intravenous or 2 mg oral doses of midazolam, respectively. VAPRISOL 30 mg/day resulted in a 3-fold increase in the AUC of simvastatin. Oral conivaptan hydrochloride 40 mg twice daily resulted in a 2-fold increase in the AUC and half-life of amlodipine [see Warnings and Precautions (5.3)].
- VAPRISOL (40 mg/day for 4 days) administered with a single 25 mg dose of warfarin, which undergoes major metabolism by CYP2C9 and minor metabolism by CYP3A, increased the mean S-warfarin AUC and S-warfarin Cmax by 14% and 17%, respectively. The corresponding prothrombin time and international normalized ratio values were unchanged.
- Captopril and Furosemide
- The pharmacokinetics of oral conivaptan (20 - 40 mg/day) were unchanged with coadministration of either captopril 25 mg or furosemide up to 80 mg/day.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Pregnancy Category C. Conivaptan has been shown to have adverse effects on the fetus when given to rats during pregnancy at systemic exposures less than those achieved at the human therapeutic dose based on AUC comparisons [see Nonclinical Toxicology (13.3)]. There are no adequate and well-controlled studies of VAPRISOL use in pregnant women. VAPRISOL should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. The patient should be apprised of the potential hazard to the fetus. Rat fetal tissue levels were < 10% of maternal plasma concentrations while placental levels were 2.2-fold higher than maternal plasma concentrations. Conivaptan that is taken up by fetal tissue is slowly cleared, suggesting that fetal accumulation is possible.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Conivaptan in women who are pregnant.
Labor and Delivery
The effect of VAPRISOL on labor and delivery in humans has not been studied. Conivaptan hydrochloride delayed delivery in rats dosed at 10 mg/kg/day by oral gavage (systemic exposure equivalent to the human therapeutic exposure based on AUC comparison). Administration of conivaptan hydrochloride at 2.5 mg/kg/day intravenously increased peripartum pup mortality (systemic exposure less than the human therapeutic exposure based on AUC comparison). These effects may be associated with conivaptan activity on oxytocin receptors in the rat. The relevance to humans is unclear [see Nonclinical Toxicology (13.3)].
Nursing Mothers
It is not known whether conivaptan is excreted in human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from VAPRISOL, a decision should be made to discontinue nursing or VAPRISOL, taking into consideration the importance of VAPRISOL to the mother. Conivaptan is excreted in milk and detected in neonates when given by intravenous administration to lactating rats. Milk levels of conivaptan in rats reached maximal levels at 1 hour post dose following intravenous administration and were up to 3 times greater than maternal plasma levels following an intravenous dose of 1 mg/kg (systemic exposure less than human therapeutic exposure based on AUC comparison).
Pediatric Use
The safety and effectiveness of VAPRISOL in pediatric patients have not been studied.
Geriatic Use
In clinical studies of VAPRISOL administered as a 20 mg IV loading dose followed by 20 mg/day or 40 mg/day IV for 2 to 4 days, 89% (20 mg/day regimen) and 60% (40 mg/day regimen) of participants were greater than or equal to 65 years of age and 60% (20 mg/day regimen) and 40% (40 mg/day regimen) were greater than or equal to 75 years of age. In general, the adverse event profile in elderly patients was similar to that seen in the general study population.
Gender
There is no FDA guidance on the use of Conivaptan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Conivaptan with respect to specific racial populations.
Renal Impairment
No clinically relevant increase in exposure was observed in subjects with mild and moderate renal impairment (CLcr 30 – 80 mL/min). No dose adjustment of VAPRISOL is necessary. Because of the high incidence of infusion site phlebitis (which can reduce vascular access sites) and unlikely benefit, use in patients with severe renal impairment (CLcr <30 mL/min) is not recommended [see Clinical Pharmacology (12.3)].
Hepatic Impairment
No clinically relevant increase in exposure was observed in subjects with mild hepatic impairment; therefore no dose adjustment of VAPRISOL is necessary. The exposure to VAPRISOL approximately doubles with moderate hepatic impairment. The impact of severe hepatic impairment on the exposure to conivaptan has not been studied [see Dosage and Administration (2.3) andClinical Pharmacology(12.3)].
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Conivaptan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Conivaptan in patients who are immunocompromised.
Administration and Monitoring
Administration
General Dosing Information
VAPRISOL is for intravenous use only.
VAPRISOL is for use in hospitalized patients only.
Administer VAPRISOL through large veins and change of the infusion site every 24 hours to minimize the risk of vascular irritation [seeWarnings and Precautions (5.5)].
Initiate with a loading dose of 20 mg IV administered over 30 minutes.
Follow the loading dose with 20 mg of VAPRISOL administered in a continuous intravenous infusion over 24 hours. After the initial day of treatment, administer VAPRISOL for an additional 1 to 3 days in a continuous infusion of 20 mg/day. If serum sodium is not rising at the desired rate, VAPRISOL may be titrated upward to a dose of 40 mg daily, administered in a continuous intravenous infusion.
The total duration of infusion of VAPRISOL (after the loading dose) should not exceed four days. The maximum daily dose of VAPRISOL (after the loading dose) is 40 mg/day.
Patients receiving VAPRISOL must have frequent monitoring of serum sodium and volume status. An overly rapid rise in serum sodium (> 12 mEq/L/24 hours) may result in serious neurologic sequelae. For patients who develop an undesirably rapid rate of rise of serum sodium, VAPRISOL should be discontinued, and serum sodium and neurologic status should be carefully monitored. If the serum sodium continues to rise, VAPRISOL should not be resumed. If hyponatremia persists or recurs, and the patient has had no evidence of neurologic sequelae of rapid rise in serum sodium, VAPRISOL may be resumed at a reduced dose [see Warnings and Precautions (5.2)]. For patients who develop hypovolemia or hypotension while receiving VAPRISOL, VAPRISOL should be discontinued, and volume status and vital signs should be frequently monitored. Once the patient is again euvolemic and is no longer hypotensive, VAPRISOL may be resumed at a reduced dose if the patient remains hyponatremic.
Preparation, Compatibility and Stability
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulate matter, discoloration or cloudiness is observed, the drug solution should not be used.
VAPRISOL is supplied in a single-use 100 mL flexible INTRAVIA Container containing a sterile premixed dilute, ready-to-use, nonpyrogenic solution of conivaptan hydrochloride, 0.2 mg per mL (20 mg/100 mL) in 5% Dextrose. NO FURTHER DILUTION OF THIS PREPARATION IS NECESSARY.
VAPRISOL is compatible with 5% Dextrose Injection. VAPRISOL is physically and chemically compatible with 0.9% Sodium Chloride Injection for up to 48 hours when the two solutions are co-administered via a Y-site connection at a flow rate for VAPRISOL of 4.2 mL/hour and at flow rates for 0.9% Sodium Chloride Injection of either 2.1 mL/hour or 6.3 mL/hour.
VAPRISOL has been shown to be incompatible with both Lactated Ringer's Injection and furosemide injection when these products are mixed in the same container; therefore, do not combine VAPRISOL with these products in the same intravenous line or container.
VAPRISOL should also not be combined with any other product in the same intravenous line or container.
Loading Dose
Administer the content of a 20 mg/100 mL VAPRISOL flexible plastic container over 30 minutes.
Continuous Infusion
For patients requiring 20 mg VAPRISOL injection per day, administer the content of one 20 mg/100 mL VAPRISOL flexible plastic container over 24 hours.
For patients requiring 40 mg VAPRISOL injection per day, administer the content of two consecutive 20 mg/100 mL VAPRISOL flexible plastic containers over 24 hours.
Since the flexible container is for single-use only, any unused portion should be discarded.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
Do not remove container from overwrap until ready for use. The overwrap is a moisture and light barrier. The inner container maintains the sterility of the product.
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. After removing overwrap, check for minute leaks by squeezing inner container firmly. If leaks are found, discard solution as sterility may be impaired. Do not use if the solution is cloudy or a precipitate is present.
DO NOT ADD SUPPLEMENTARY MEDICATION.
Preparation for Administration:
- Suspend container from eyelet support.
- Remove protector from outlet port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
Hepatic Impairment
In patients with moderate hepatic impairment, initiate VAPRISOL with a loading dose of 10 mg over 30 minutes followed by 10 mg per day as a continuous infusion for 2 to 4 days. If serum sodium is not rising at the desired rate, VAPRISOL may be titrated upward to 20 mg per day [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Monitoring
There is limited information regarding Conivaptan Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Conivaptan and IV administrations.
Overdosage
There is limited information regarding Conivaptan overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Conivaptan Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Conivaptan Mechanism of Action in the drug label.
Structure
There is limited information regarding Conivaptan Structure in the drug label.
Pharmacodynamics
There is limited information regarding Conivaptan Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Conivaptan Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Conivaptan Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Conivaptan Clinical Studies in the drug label.
How Supplied
There is limited information regarding Conivaptan How Supplied in the drug label.
Storage
There is limited information regarding Conivaptan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Conivaptan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Conivaptan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Conivaptan Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Conivaptan interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Conivaptan Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Conivaptan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.