Conivaptan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
:: (Dosage) | :: (Dosage) | ||
|contraindications=* Hypovolemic Hyponatremia | |contraindications=* Hypovolemic Hyponatremia | ||
:* VAPRISOL is contraindicated in patients with hypovolemic [[hyponatremia]]. | :* VAPRISOL is contraindicated in patients with hypovolemic [[hyponatremia]]. | ||
| Line 61: | Line 60: | ||
In clinical trials where VAPRISOL was administered to 79 hypervolemic hyponatremic patients with underlying heart failure and intravenous placebo administered to 10 patients, adverse cardiac failure events, atrial dysrhythmias, and sepsis occurred more frequently among patients treated with VAPRISOL (32%, 5% and 8% respectively) than among patients treated with placebo (20%, 0% and 0% respectively) [see Warnings and Precautions (5.1)]. | In clinical trials where VAPRISOL was administered to 79 hypervolemic hyponatremic patients with underlying heart failure and intravenous placebo administered to 10 patients, adverse cardiac failure events, atrial dysrhythmias, and sepsis occurred more frequently among patients treated with VAPRISOL (32%, 5% and 8% respectively) than among patients treated with placebo (20%, 0% and 0% respectively) [see Warnings and Precautions (5.1)]. | ||
|drugInteractions= | |drugInteractions=* CYP3A | ||
Conivaptan is a sensitive substrate of CYP3A. The effect of ketoconazole, a potent CYP3A inhibitor, on the pharmacokinetics of intravenous conivaptan has not been evaluated. Coadministration of oral conivaptan hydrochloride 10 mg with ketoconazole 200 mg resulted in 4- and 11-fold increases in Cmax and AUC of conivaptan, respectively [see Contraindications (4.2)]. | :* Conivaptan is a sensitive substrate of CYP3A. The effect of [[ketoconazole]], a potent CYP3A inhibitor, on the pharmacokinetics of intravenous conivaptan has not been evaluated. Coadministration of oral conivaptan [[hydrochloride]] 10 mg with [[ketoconazole]] 200 mg resulted in 4- and 11-fold increases in Cmax and AUC of conivaptan, respectively [see Contraindications (4.2)]. | ||
Conivaptan is a potent mechanism-based inhibitor of CYP3A. The effect of conivaptan on the pharmacokinetics of co-administered CYP3A substrates has been evaluated with the coadministration of conivaptan with midazolam, simvastatin, and amlodipine. VAPRISOL 40 mg/day increased the mean AUC values by approximately 2- and 3-fold for 1 mg intravenous or 2 mg oral doses of midazolam, respectively. VAPRISOL 30 mg/day resulted in a 3-fold increase in the AUC of simvastatin. Oral conivaptan hydrochloride 40 mg twice daily resulted in a 2-fold increase in the AUC and half-life of amlodipine [see Warnings and Precautions (5.3)]. | :* Conivaptan is a potent mechanism-based inhibitor of CYP3A. The effect of conivaptan on the pharmacokinetics of co-administered CYP3A substrates has been evaluated with the coadministration of conivaptan with [[midazolam]], [[simvastatin]], and [[amlodipine]]. VAPRISOL 40 mg/day increased the mean AUC values by approximately 2- and 3-fold for 1 mg intravenous or 2 mg oral doses of [[midazolam]], respectively. VAPRISOL 30 mg/day resulted in a 3-fold increase in the AUC of [[simvastatin]]. Oral conivaptan hydrochloride 40 mg twice daily resulted in a 2-fold increase in the AUC and half-life of [[amlodipine]] [see Warnings and Precautions (5.3)]. | ||
Coadministration of a 0.5 mg dose of digoxin, a P-glycoprotein substrate, with oral conivaptan hydrochloride 40 mg twice daily resulted in a 30% reduction in clearance and 79% and 43% increases in digoxin Cmax and AUC values, respectively [see Warnings and Precautions (5.4)]. | * [[Digoxin]] | ||
:* Coadministration of a 0.5 mg dose of [[digoxin]], a P-glycoprotein substrate, with oral conivaptan hydrochloride 40 mg twice daily resulted in a 30% reduction in clearance and 79% and 43% increases in [[digoxin]] Cmax and AUC values, respectively [see Warnings and Precautions (5.4)]. | |||
VAPRISOL (40 mg/day for 4 days) administered with a single 25 mg dose of warfarin, which undergoes major metabolism by CYP2C9 and minor metabolism by CYP3A, increased the mean S-warfarin AUC and S-warfarin Cmax by 14% and 17%, respectively. The corresponding prothrombin time and international normalized ratio values were unchanged. | |||
* [[Warfarin]] | |||
The pharmacokinetics of oral conivaptan (20 - 40 mg/day) were unchanged with coadministration of either captopril 25 mg or furosemide up to 80 mg/day. | :* VAPRISOL (40 mg/day for 4 days) administered with a single 25 mg dose of [[warfarin]], which undergoes major metabolism by CYP2C9 and minor metabolism by CYP3A, increased the mean [[S-warfarin]] AUC and [[S-warfarin]] Cmax by 14% and 17%, respectively. The corresponding prothrombin time and international normalized ratio values were unchanged. | ||
* [[Captopril]] and [[Furosemide]] | |||
:* The pharmacokinetics of oral conivaptan (20 - 40 mg/day) were unchanged with coadministration of either [[captopril]] 25 mg or [[furosemide]] up to 80 mg/day. | |||
}} | }} | ||
Revision as of 15:09, 22 April 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Conivaptan is a Vasopressin antagonist that is FDA approved for the {{{indicationType}}} of raising serum sodium in hospitalized patients with euvolemic and hypervolemic hyponatremia.. Common adverse reactions include hypertension, orthostatic hypotension, peripheral edema, phlebitis, injection site reaction, hypokalemia, increased thirst, constipation, diarrhea, vomiting, headache, increased frequency of urination, polyuria, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
<h4>Condition 1</h4>
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Conivaptan FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Hypovolemic Hyponatremia
- VAPRISOL is contraindicated in patients with hypovolemic hyponatremia.
- Coadministration with Potent CYP3A Inhibitors
- The coadministration of VAPRISOL with potent CYP3A inhibitors, such as ketoconazole, itraconazole, clarithromycin, ritonavir, and indinavir, is contraindicated [see Drug Interactions (7.1)].
- Anuric Patients
- In patients unable to make urine, no benefit can be expected [see Clinical Pharmacology (12.3)].
- Known Allergy to Corn or Corn Products
- Solutions containing dextrose are contraindicated in patients with known allergy to corn or corn products.
Warnings
- Hypovolemic hyponatremia
- VAPRISOL is contraindicated in patients with hypovolemic hyponatremia.
- Coadministration with Potent CYP3A Inhibitors
- The coadministration of VAPRISOL with potent CYP3A inhibitors, such as ketoconazole, itraconazole, clarithromycin, ritonavir, and indinavir, is contraindicated [see Drug Interactions (7.1)].
- Anuric Patients
- In patients unable to make urine, no benefit can be expected [see Clinical Pharmacology (12.3)].
- Known Allergy to Corn or Corn Products
- Solutions containing dextrose are contraindicated in patients with known allergy to corn or corn products.
- hyponatremia Associated with Heart Failure
- The amount of safety data on the use of VAPRISOL in patients with hypervolemic hyponatremia associated with heart failure is limited. VAPRISOL should be used to raise serum sodium in such patients only after consideration of other treatment options [see Adverse Reactions (6.1)].
- Overly Rapid Correction of Serum Sodium
- Osmotic demyelination syndrome is a risk associated with overly rapid correction of hyponatremia (i.e., > 12 mEq/L/24 hours). Osmotic demyelination results in dysarthria, mutism, dysphagia, lethargy, affective changes, spastic quadriparesis, seizures, coma or death. In susceptible patients, including those with severe malnutrition, alcoholism or advanced liver disease, use slower rates of correction. In controlled clinical trials of VAPRISOL, about 9% of patients who received VAPRISOL in doses of 20-40 mg/day IV had rises of serum sodium >12 mEq/L/24 hours, but none of these patients had evidence of osmotic demyelination or permanent neurologic sequelae. Serum sodium concentration and neurologic status should be monitored appropriately during VAPRISOL administration, and VAPRISOL administration should be discontinued if the patient develops an undesirably rapid rate of rise of serum sodium. If the serum sodium concentration continues to rise, VAPRISOL should not be resumed. If hyponatremia persists or recurs (after initial discontinuation of VAPRISOL for an undesirably rapid rate of rise of serum sodium concentration), and the patient has had no evidence of neurologic sequelae of rapid rise in serum sodium, VAPRISOL may be resumed at a reduced dose [see Dosage and Administration (2.1)].
- Coadministration of VAPRISOL and Drugs Eliminated Primarily by CYP3A Mediated Metabolism
- In clinical trials of oral conivaptan, two cases of rhabdomyolysis occurred in patients who were also receiving a CYP3A-metabolized HMG-CoA reductase inhibitor. Avoid concomitant use of VAPRISOL with drugs eliminated primarily by CYP3A-mediated metabolism. Subsequent treatment with CYP3A substrate drugs may be initiated no sooner than 1 week after the infusion of VAPRISOL is completed [see Drug Interactions (7.1)].
- Coadministration of VAPRISOL and digoxin
- Infusion Site Reactions
- Infusion site reactions are common and can include serious reactions, even with proper infusion rates [see Adverse Reactions (6.1)]. Administer VAPRISOL via large veins, and rotate the infusion site every 24 hours [see Dosage and Administration (2.1)].
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse event information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates. The most common adverse reactions reported with VAPRISOL administration were infusion site reactions. In studies in patients and healthy volunteers, infusion site reactions occurred in 73% and 63% of subjects treated with VAPRISOL 20 mg/day and 40 mg/day, respectively, compared to 4% in the placebo group. Infusion site reactions were the most common type of adverse event leading to discontinuation of VAPRISOL. Discontinuations from treatment due to infusion site reactions were more common among VAPRISOL-treated patients (3%) than among placebo-treated patients (0%). Some serious infusion site reactions did occur [see Dosage and Administration (2.1) and Warnings and Precautions (5.5)]. The adverse reactions presented in Table 1 are derived from 72 healthy volunteers and 243 patients with euvolemic or hypervolemic hyponatremia who received VAPRISOL 20 mg IV as a loading dose followed by 40 mg/day IV for 2 to 4 days, from 37 patients with euvolemic or hypervolemic hyponatremia who received VAPRISOL 20 mg IV as a loading dose followed by 20 mg/day IV for 2 to 4 days in an open-label study, and from 40 healthy volunteers and 29 patients with euvolemic or hypervolemic hyponatremia who received placebo. The adverse reactions occurred in at least 5% of patients treated with VAPRISOL and at a higher incidence for VAPRISOL-treated patients than for placebo-treated patients.
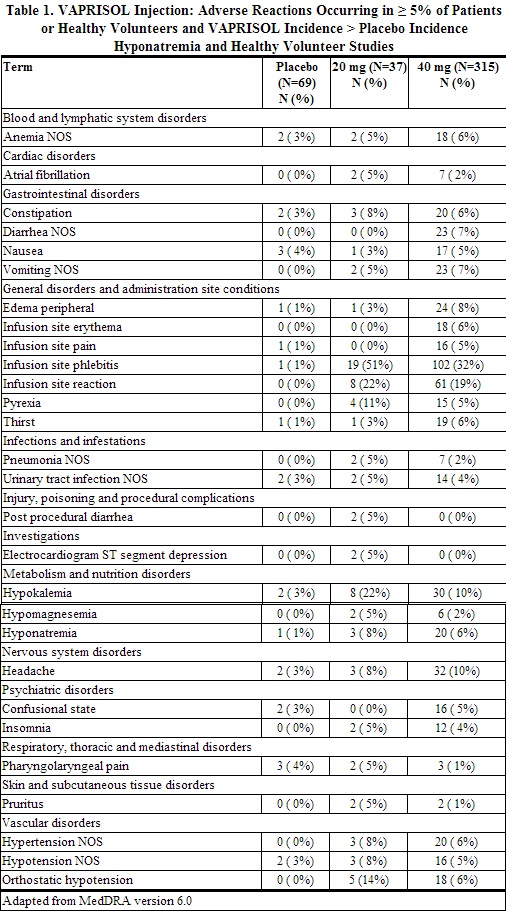
Although a dose of 80 mg/day of VAPRISOL was also studied, it was associated with a higher incidence of infusion site reactions and a higher rate of discontinuation for adverse events than was the 40 mg/day VAPRISOL dose. The maximum recommended daily dose of VAPRISOL (after the loading dose) is 40 mg/day.
Heart failure with hypervolemic hyponatremia
In clinical trials where VAPRISOL was administered to 79 hypervolemic hyponatremic patients with underlying heart failure and intravenous placebo administered to 10 patients, adverse cardiac failure events, atrial dysrhythmias, and sepsis occurred more frequently among patients treated with VAPRISOL (32%, 5% and 8% respectively) than among patients treated with placebo (20%, 0% and 0% respectively) [see Warnings and Precautions (5.1)].
Postmarketing Experience
There is limited information regarding Conivaptan Postmarketing Experience in the drug label.
Drug Interactions
- CYP3A
- Conivaptan is a sensitive substrate of CYP3A. The effect of ketoconazole, a potent CYP3A inhibitor, on the pharmacokinetics of intravenous conivaptan has not been evaluated. Coadministration of oral conivaptan hydrochloride 10 mg with ketoconazole 200 mg resulted in 4- and 11-fold increases in Cmax and AUC of conivaptan, respectively [see Contraindications (4.2)].
- Conivaptan is a potent mechanism-based inhibitor of CYP3A. The effect of conivaptan on the pharmacokinetics of co-administered CYP3A substrates has been evaluated with the coadministration of conivaptan with midazolam, simvastatin, and amlodipine. VAPRISOL 40 mg/day increased the mean AUC values by approximately 2- and 3-fold for 1 mg intravenous or 2 mg oral doses of midazolam, respectively. VAPRISOL 30 mg/day resulted in a 3-fold increase in the AUC of simvastatin. Oral conivaptan hydrochloride 40 mg twice daily resulted in a 2-fold increase in the AUC and half-life of amlodipine [see Warnings and Precautions (5.3)].
- VAPRISOL (40 mg/day for 4 days) administered with a single 25 mg dose of warfarin, which undergoes major metabolism by CYP2C9 and minor metabolism by CYP3A, increased the mean S-warfarin AUC and S-warfarin Cmax by 14% and 17%, respectively. The corresponding prothrombin time and international normalized ratio values were unchanged.
- Captopril and Furosemide
- The pharmacokinetics of oral conivaptan (20 - 40 mg/day) were unchanged with coadministration of either captopril 25 mg or furosemide up to 80 mg/day.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Conivaptan in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Conivaptan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Conivaptan during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Conivaptan in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Conivaptan in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Conivaptan in geriatric settings.
Gender
There is no FDA guidance on the use of Conivaptan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Conivaptan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Conivaptan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Conivaptan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Conivaptan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Conivaptan in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Conivaptan Administration in the drug label.
Monitoring
There is limited information regarding Conivaptan Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Conivaptan and IV administrations.
Overdosage
There is limited information regarding Conivaptan overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Conivaptan Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Conivaptan Mechanism of Action in the drug label.
Structure
There is limited information regarding Conivaptan Structure in the drug label.
Pharmacodynamics
There is limited information regarding Conivaptan Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Conivaptan Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Conivaptan Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Conivaptan Clinical Studies in the drug label.
How Supplied
There is limited information regarding Conivaptan How Supplied in the drug label.
Storage
There is limited information regarding Conivaptan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Conivaptan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Conivaptan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Conivaptan Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Conivaptan interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Conivaptan Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Conivaptan Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.