Colorectal cancer pathophysiology
|
Colorectal cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Colorectal cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Colorectal cancer pathophysiology |
|
Risk calculators and risk factors for Colorectal cancer pathophysiology |
To view the pathophysiology of familial adenomatous polyposis (FAP), click here
To view the pathophysiology of hereditary nonpolyposis colorectal cancer (HNPCC), click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Saarah T. Alkhairy, M.D.; Elliot B. Tapper, M.D.
Overview
The pathogenesis of colorectal carcinoma (CRC) involves genetic instability, epigenetic alteration, chronic inflammation, oxidative stress, and intestinal microbiota. Right-sided and left-sided tumors differ in their gross pathology. Depending on glandular architecture, cellular pleomorphism, and mucosecretion of the predominant pattern, adenocarcinoma may present in three degrees of differentiation: well, moderately, and poorly differentiated.
Pathogenesis
Sporadic colorectal cancers
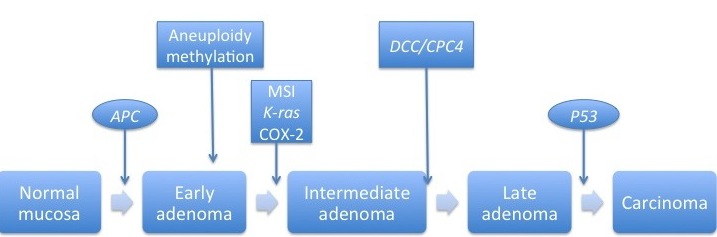
The picture below depicts the molecular pathogenesis of sporadic colon cancer[1].
Sporadic colorectal cancer originates from the epithelial cells that line the colon or rectum. It may involve the following[2]:
- The APC gene
- It produces the APC protein, which prevents the accumulation of β-cateninprotein (responsible for stem cell renewal)
- If there is a mutation of the APC protein, β-cateninprotein accumulates and causes inappropriately high levels of stem cell renewal
- It produces the APC protein, which prevents the accumulation of β-cateninprotein (responsible for stem cell renewal)
- The TP53 gene
- It produces the p53 protein, which monitors cell division and promotes apoptosis if there are cell defects
- If there is a mutation, there is no control over cell division or apoptosis
- TGF-β and DCC (Deleted in Colorectal Cancer)
- Both of these proteins are responsible for apoptosis, but are deactivated in CRC
- Oncogenes
- These genes stimulate the cell to divide
- If there is a mutation of an oncogene, there may be an over-activation of cell proliferation
- Examples are K-RAS and RAF
Colitis-associated colorectal cancers
The picture below depicts the molecular pathogenesis of colitis-assocated colon cancer[1].
At a microbiological level, the development of colitis-associated colorectal cancers (CRC) can be linked to defects within the cell cycle[3]. Although it is poorly understood, the following five factors may be responsible for its neoplastic changes[1]:
- Genetic instability
- Epigenetic alteration
- Chronic inflammation
- Oxidative stress
- Intestinal microbiota
Genetic instability
- Aneuploidy is present in approximately 50%-90% of cancers[1]
- A loss of the P53 function is common in colitis-associated CRC, although it can be found in sporadic colon cancer[1]
- A loss of the adenomatous polyposis (APC) function is common in sporadic CRC, although it can be found in colitis-associated colon cancer[1]
- The following are two types of genomic instability[4]
- Chromosomal instability (CIN) occurs when either whole chromosomes or parts of chromosomes are duplicated or deleted; it has a 85% frequency
- Microsatellite instability (MSI) is the condition of genetic hypermutability that results from impaired DNA mismatch repair; it has a 15% frequency
Epigenetic alteration
- Sporadic CRC can develop from dysplasia in 1 or 2 foci of the colon[5][6]
- Colitis-associated CRC can develop from multifocal dysplasia[5][6]
- This indicates a field change effect where large areas of cells within the colon are affected by carcinogenic alterations
Chronic inflammation
- COX-2 is triggered by inflammatory stimuli such as IL-1, IFN-γ, and TNF-α[7]
- COX-2 expression is elevated in approximately 85% of adenocarcinomas[7]
Oxidative stress
- Oxidative stress results from inflammatory reactions which include inflammatory cells, activated neutrophils, and macrophages
- Macrophages produce large amounts of reactive oxygen and nitrogen species (RONS)[8]
- RONs can interact with key genes involved in carcinogenic pathways such as P53 and DNA mismatch repair genes[8]
Intestinal microbiota
- The Modification of enteric flora by probiotic lactobacilli is a proposed mechanism may contribute to the development of colitis-associated cancer[9]
Genetics
CRC can be grouped into three categories from a genetic perspective[10]:
- Sporadic (75% of cases) - no apparent indication of a hereditary component
- Familial (20% of cases) - multifactorial hereditary factors or common exposures to non-genetic risk factors or both
- Hereditary (10% of cases)
- Hereditary nonpolyposis colon cancer (HNPCC) also known as Lynch Syndrome results from mutations in hMLH1, hMSH2, hMSH6, and PMS2
- Familial adenomatous polyposis (FAP) results from mutations in the APC gene located on chromosome 5p22.2
- MUTYH-associated polyposis (MAP) results from biallelic mutation of the MutY, E. Coli, Homolog gene which functions to remove adenine residues mispaired with 8-hydroxyguanine in DNA
Gross Pathology
- Right-sided tumors (ascending colon and cecum) tends to grow outwards from one location in the bowel wall (exophytic)
- Left-sided tumours tend to be circumferential

Microscopic Pathology
- Tumor cells form irregular tubular structures, harboring pleuristratification, multiple lumens, and reduced stroma
- Sometimes, tumor cells are discohesive and secrete mucus, which invades the interstitium producing large pools of mucus/colloid (optically "empty" spaces)
- If the mucus remains inside the tumor cell, it pushes the nucleus at the periphery (signet-ring cell)
- Depending on glandular architecture, cellular pleomorphism, and mucosecretion of the predominant pattern, adenocarcinoma may present in one of three degrees of differentiation: well, moderately, or poorly differentiated[11]

Grades of Colorectal Cancer
The grade describes how closely the cancer looks like normal tissue when seen under a microscope. This is sometimes used to distinguish whether a patient should get adjuvant treatment with chemotherapy after surgery.
- Grade 1 - Well differentiated
- Grade 2 - Moderately differentiated
- Grade 3 - Poorly differentiated
- Grade 4 - Undifferentiated
Video
{{#ev:youtube|Sh65aXndqXk}}
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Kim, Eun Ran (2014). "Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis". World Journal of Gastroenterology. 20 (29): 9872. doi:10.3748/wjg.v20.i29.9872. ISSN 1007-9327.
- ↑ Markowitz SD, Bertagnolli MM (2009). "Molecular origins of cancer: Molecular basis of colorectal cancer". N Engl J Med. 361 (25): 2449–60. doi:10.1056/NEJMra0804588. PMC 2843693. PMID 20018966.
- ↑ Scully R (2010). "The spindle-assembly checkpoint, aneuploidy, and gastrointestinal cancer". The New England Journal of Medicine. 363 (27): 2665–6. doi:10.1056/NEJMe1008017. PMID 21190461. Retrieved 2011-12-12. Unknown parameter
|month=ignored (help) - ↑ Zivić R, Bjelaković G, Koraćević D (1975). "[Amino acid constitution of the urine in children with rheumatic fever]". Reumatizam. 22 (1): 21–5. PMID 1118685.
- ↑ 5.0 5.1 Kraus S, Arber N (2009). "Inflammation and colorectal cancer". Curr Opin Pharmacol. 9 (4): 405–10. doi:10.1016/j.coph.2009.06.006. PMID 19589728.
- ↑ 6.0 6.1 Itzkowitz S (2003). "Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice". J Clin Gastroenterol. 36 (5 Suppl): S70–4, discussion S94-6. PMID 12702969.
- ↑ 7.0 7.1 Elzagheid A, Emaetig F, Alkikhia L, Buhmeida A, Syrjänen K, El-Faitori O; et al. (2013). "High cyclooxygenase-2 expression is associated with advanced stages in colorectal cancer". Anticancer Res. 33 (8): 3137–43. PMID 23898071.
- ↑ 8.0 8.1 Ullman TA, Itzkowitz SH (2011). "Intestinal inflammation and cancer". Gastroenterology. 140 (6): 1807–16. doi:10.1053/j.gastro.2011.01.057. PMID 21530747.
- ↑ O'Mahony L, Feeney M, O'Halloran S, Murphy L, Kiely B, Fitzgibbon J; et al. (2001). "Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice". Aliment Pharmacol Ther. 15 (8): 1219–25. PMID 11472326.
- ↑ Schlussel AT, Gagliano RA, Seto-Donlon S, Eggerding F, Donlon T, Berenberg J; et al. (2014). "The evolution of colorectal cancer genetics-Part 1: from discovery to practice". J Gastrointest Oncol. 5 (5): 326–35. doi:10.3978/j.issn.2078-6891.2014.069. PMC 4173047. PMID 25276405.
- ↑ Pathology atlas (in Romanian)