Clonazepam
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clonazepam is a Benzodiazepine that is FDA approved for the {{{indicationType}}} of Lennox-Gastaut syndrome (petit mal variant), akinetic and myoclonic seizures, panic disorder, with or without agoraphobia. Common adverse reactions include Ataxia,Coordination problem,Dizziness,Somnolence,Problem behavior,Upper respiratory infection,Fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Seizure Disorders
- Dosing information
- Adults
- Initial dose: not exceed 1.5 mg PO tid. Dosage may be increased in increments of 0.5 mg to 1 mg every 3 days until seizures are adequately controlled or until side effects preclude any further increase. Maintenance dosage must be individualized for each patient depending upon response. Maximum recommended daily dose is 20 mg.
- The use of multiple anticonvulsants may result in an increase of depressant adverse effects. This should be considered before adding clonazepam tablets to an existing anticonvulsant regimen.
- Geriatric Patients
- There is no clinical trial experience with clonazepam tablets in seizure disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam tablets and observed closely
Panic disorder
- Dosing införmation
- Adults
- Initial dose: 0.25 mg bid. An increase to the target dose for most patients of 1 mg/day may be made after 3 days.
- Recommended dose: 1 mg/day is based on the results from a fixed dose study in which the optimal effect was seen at 1 mg/day. Higher doses of 2 mg/day, 3 mg/day and 4 mg/day in that study were less effective than the 1 mg/day dose and were associated with more adverse effects.
- Maximum dosage: 4 mg/day, and in those instances, the dose may be increased in increments of 0.125 mg to 0.25 mg bid every 3 days until panic disorder is controlled or until side effects make further increases undesired. To reduce the inconvenience of somnolence, administration of one dose at bedtime may be desirable.
- Treatment should be discontinued gradually, with a decrease of 0.125 mg bid every 3 days, until the drug is completely withdrawn.
- There is no body of evidence available to answer the question of how long the patient treated with clonazepam should remain on it. Therefore, the physician who elects to use clonazepam tablets for extended periods should periodically reevaluate the long-term usefulness of the drug for the individual patient.
- Geriatric Patients
- There is no clinical trial experience with clonazepam tablets in panic disorder patients 65 years of age and older. In general, elderly patients should be started on low doses of clonazepam tablets and observed closely.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clonazepam in adult patients.
Non–Guideline-Supported Use
Restless legs syndrome
- Dosing information
- 0.5 to 2 mg at bedtime[1]
Sleep walking disorder
- Dosing information
- 0.25 to 2 mg at bedtime[2]
Social phobia
- Dosing information
- 1 to 2.5 mg daily [3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Seizure disorder
- Dosing information
- lonazepam tablets are administered orally. In order to minimize drowsiness, the initial dose for infants and children (up to 10 years of age or 30 kg of body weight) should be between 0.01 mg/kg/day and 0.03 mg/kg/day but not to exceed 0.05 mg/kg/day given in two or three divided doses. Dosage should be increased by no more than 0.25 mg to 0.5 mg every third day until a daily maintenance dose of 0.1 mg/kg to 0.2 mg/kg of body weight has been reached, unless seizures are controlled or side effects preclude further increase. Whenever possible, the daily dose should be divided into three equal doses. If doses are not equally divided, the largest dose should be given before retiring.
Panic disorder
- Dosing information
- There is no clinical trial experience with clonazepam tablets in panic disorder patients under 18 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clonazepam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clonazepam in pediatric patients.
Contraindications
Clonazepam should not be used in patients with a history of sensitivity to benzodiazepines, nor in patients with clinical or biochemical evidence of significant liver disease. It may be used in patients with open angle glaucoma who are receiving appropriate therapy but is contraindicated in acute narrow angle glaucoma.
Warnings
Interference With Cognitive and Motor Performance Since clonazepam produces CNS depression, patients receiving this drug should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating machinery or driving a motor vehicle. They should also be warned about the concomitant use of alcohol or other CNS-depressant drugs during clonazepam therapy (see PRECAUTIONS,Drug InteractionsDrug Interactions and PRECAUTIONS, Information for Patients). Suicidal Behavior and Ideation Antiepileptic drugs (AEDs), including clonazepam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43% compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide. The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed. The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
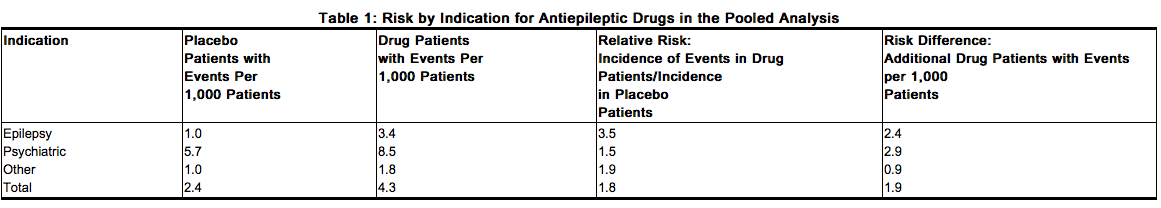
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications. Anyone considering prescribing clonazepam or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated. Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Pregnancy Risks
Data from several sources raise concerns about the use of clonazepam during pregnancy.
Animal Findings
In three studies in which clonazepam was administered orally to pregnant rabbits at doses of 0.2 mg/kg/day, 1 mg/kg/day, 5 mg/kg/day or 10 mg/kg/day (low dose approximately 0.2 times the maximum recommended human dose of 20 mg/day for seizure disorders and equivalent to the maximum dose of 4 mg/day for panic disorder, on a mg/m2 basis) during the period of organogenesis, a similar pattern of malformations (cleft palate, open eyelid, fused sternebrae and limb defects) was observed in a low, non-dose-related incidence in exposed litters from all dosage groups. Reductions in maternal weight gain occurred at dosages of 5 mg/kg/day or greater and reduction in embryo-fetal growth occurred in one study at a dosage of 10 mg/kg/day. No adverse maternal or embryo-fetal effects were observed in mice and rats following administration during organogenesis of oral doses up to 15 mg/kg/day or 40 mg/kg/day, respectively (4 and 20 times the maximum recommended human dose of 20 mg/day for seizure disorders and 20 and 100 times the maximum dose of 4 mg/day for panic disorder, respectively, on a mg/m2 basis).
General Concerns and Considerations About Anticonvulsants
Recent reports suggest an association between the use of anticonvulsant drugs by women with epilepsy and an elevated incidence of birth defects in children born to these women. Data are more extensive with respect to diphenylhydantoin and phenobarbital, but these are also the most commonly prescribed anticonvulsants; less systematic or anecdotal reports suggest a possible similar association with the use of all known anticonvulsant drugs. In children of women treated with drugs for epilepsy, reports suggesting an elevated incidence of birth defects cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; the possibility also exists that other factors, (e.g., genetic factors or the epileptic condition itself), may be more important than drug therapy in leading to birth defects. The great majority of mothers on anticonvulsant medication deliver normal infants. It is important to note that anticonvulsant drugs should not be discontinued in patients in whom the drug is administered to prevent seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy; however, it cannot be said with any confidence that even mild seizures do not pose some hazards to the developing embryo or fetus.
General Concerns About Benzodiazepines
An increased risk of congenital malformations associated with the use of benzodiazepine drugs has been suggested in several studies. There may also be non-teratogenic risks associated with the use of benzodiazepines during pregnancy. There have been reports of neonatal flaccidity, respiratory and feeding difficulties, and hypothermia in children born to mothers who have been receiving benzodiazepines late in pregnancy. In addition, children born to mothers receiving benzodiazepines late in pregnancy may be at some risk of experiencing withdrawal symptoms during the postnatal period. Advice Regarding the Use of Clonazepam in Women of Childbearing Potential In general, the use of clonazepam in women of childbearing potential, and more specifically during known pregnancy, should be considered only when the clinical situation warrants the risk to the fetus. The specific considerations addressed above regarding the use of anticonvulsants for epilepsy in women of childbearing potential should be weighed in treating or counseling these women. Because of experience with other members of the benzodiazepine class, clonazepam is assumed to be capable of causing an increased risk of congenital abnormalities when administered to a pregnant woman during the first trimester. Because use of these drugs is rarely a matter of urgency in the treatment of panic disorder, their use during the first trimester should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Patients should also be advised that if they become pregnant during therapy or intend to become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
Withdrawal Symptoms
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines
==PRECAUTIONS==
===General===
Worsening of Seizures
When used in patients in whom several different types of seizure disorders coexist, clonazepam may increase the incidence or precipitate the onset of generalized tonic-clonic seizures (grand mal). This may require the addition of appropriate anticonvulsants or an increase in their dosages. The concomitant use of valproic acid and clonazepam may produce absence status.
Laboratory Testing During Long-Term Therapy
Periodic blood counts and liver function tests are advisable during long-term therapy with clonazepam.
Risks of Abrupt Withdrawal
The abrupt withdrawal of clonazepam, particularly in those patients on long-term, high-dose therapy, may precipitate status epilepticus. Therefore, when discontinuing clonazepam, gradual withdrawal is essential. While clonazepam is being gradually withdrawn, the simultaneous substitution of another anticonvulsant may be indicated. Caution in Renally Impaired Patients Metabolites of clonazepam are excreted by the kidneys; to avoid their excess accumulation, caution should be exercised in the administration of the drug to patients with impaired renal function.
Hypersalivation
Clonazepam may produce an increase in salivation. This should be considered before giving the drug to patients who have difficulty handling secretions. Because of this and the possibility of respiratory depression, clonazepam should be used with caution in patients with chronic respiratory diseases.
Adverse Reactions
Clinical Trials Experience
The adverse experiences for clonazepam are provided separately for patients with seizure disorders and with panic disorder.
Seizure Disorders
The most frequently occurring side effects of clonazepam are referable to CNS depression. Experience in treatment of seizures has shown that drowsiness has occurred in approximately 50% of patients and ataxia in approximately 30%. In some cases, these may diminish with time; behavior problems have been noted in approximately 25% of patients. Others, listed by system, are: Neurologic: Abnormal eye movements, aphonia, choreiform movements, coma, diplopia, dysarthria, dysdiadochokinesis, “glassy-eyed” appearance, headache, hemiparesis, hypotonia, nystagmus, respiratory depression, slurred speech, tremor, vertigo Psychiatric: Confusion, depression, amnesia, hallucinations, hysteria, increased libido, insomnia, psychosis (the behavior effects are more likely to occur in patients with a history of psychiatric disturbances). The following paradoxical reactions have been observed: excitability, irritability, aggressive behavior, agitation, nervousness, hostility, anxiety, sleep disturbances, nightmares and vivid dreams Respiratory: Chest congestion, rhinorrhea, shortness of breath, hypersecretion in upper respiratory passages Cardiovascular: Palpitations Dermatologic: Hair loss, hirsutism, skin rash, ankle and facial edema Gastrointestinal: Anorexia, coated tongue, constipation, diarrhea, dry mouth, encopresis, gastritis, increased appetite, nausea, sore gums Genitourinary: Dysuria, enuresis, nocturia, urinary retention Musculoskeletal: Muscle weakness, pains Miscellaneous: Dehydration, general deterioration, fever, lymphadenopathy, weight loss or gain Hematopoietic: Anemia, leukopenia, thrombocytopenia, eosinophilia Hepatic: Hepatomegaly, transient elevations of serum transaminases and alkaline phosphatase
Panic Disorder
Adverse events during exposure to clonazepam were obtained by spontaneous report and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, CIGY dictionary terminology has been used to classify reported adverse events, except in certain cases in which redundant terms were collapsed into more meaningful terms, as noted below. The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation. Adverse Findings Observed in Short-Term, Placebo Controlled Trials Adverse Events Associated With Discontinuation of Treatment Overall, the incidence of discontinuation due to adverse events was 17% in clonazepam compared to 9% for placebo in the combined data of two 6- to 9-week trials. The most common events (≥1%) associated with discontinuation and a dropout rate twice or greater for clonazepam than that of placebo included the following:
Postmarketing Experience
There is limited information regarding Clonazepam Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Clonazepam Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Clonazepam in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clonazepam in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clonazepam during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Clonazepam in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Clonazepam in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Clonazepam in geriatric settings.
Gender
There is no FDA guidance on the use of Clonazepam with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clonazepam with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clonazepam in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clonazepam in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clonazepam in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clonazepam in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Clonazepam Administration in the drug label.
Monitoring
There is limited information regarding Clonazepam Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Clonazepam and IV administrations.
Overdosage
There is limited information regarding Clonazepam overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Clonazepam Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Clonazepam Mechanism of Action in the drug label.
Structure
There is limited information regarding Clonazepam Structure in the drug label.
Pharmacodynamics
There is limited information regarding Clonazepam Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Clonazepam Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Clonazepam Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Clonazepam Clinical Studies in the drug label.
How Supplied
There is limited information regarding Clonazepam How Supplied in the drug label.
Storage
There is limited information regarding Clonazepam Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Clonazepam |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clonazepam |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clonazepam Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Clonazepam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Clonazepam Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Clonazepam Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Peled R, Lavie P (1987). "Double-blind evaluation of clonazepam on periodic leg movements in sleep". J Neurol Neurosurg Psychiatry. 50 (12): 1679–81. PMC 1032613. PMID 3437302.
- ↑ Schenck CH, Milner DM, Hurwitz TD, Bundlie SR, Mahowald MW (1989). "A polysomnographic and clinical report on sleep-related injury in 100 adult patients". Am J Psychiatry. 146 (9): 1166–73. PMID 2764174.
- ↑ Connor KM, Davidson JR, Potts NL, Tupler LA, Miner CM, Malik ML; et al. (1998). "Discontinuation of clonazepam in the treatment of social phobia". J Clin Psychopharmacol. 18 (5): 373–8. PMID 9790154.