Clobazam
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clobazam is a Benzodiazepine that is FDA approved for the treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older. Common adverse reactions include constipation, somnolence or sedation, pyrexia, lethargy, and drooling.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Seizures associated with Lennox-Gastaut syndrome (LGS)
- A daily dose of ONFI greater than 5 mg should be administered in divided doses twice daily
- 5 mg daily dose can be administered as a single dose.
- Dose patients according to body weight.
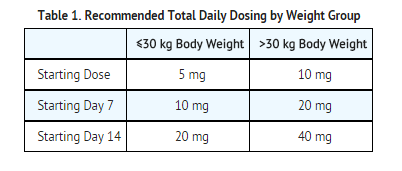
- Do not proceed with dose escalation more rapidly than weekly, because serum concentrations of clobazam and its active metabolite require 5 and 9 days, respectively, to reach steady-state.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clobazam in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clobazam in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Seizures associated with Lennox-Gastaut syndrome (LGS) (2 years of age or older)
- A daily dose of ONFI greater than 5 mg should be administered in divided doses twice daily
- 5 mg daily dose can be administered as a single dose.
- Dose patients according to body weight.

- Do not proceed with dose escalation more rapidly than weekly, because serum concentrations of clobazam and its active metabolite require 5 and 9 days, respectively, to reach steady-state.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clobazam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clobazam in pediatric patients.
Contraindications
- ONFI is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients.
- Hypersensitivity reactions have included serious dermatological reactions.
Warnings
Somnolence or Sedation
- ONFI causes somnolence and sedation.
- In clinical trials, somnolence or sedation was reported at all effective doses and was dose-related.
- In general, somnolence and sedation begin within the first month of treatment and may diminish with continued treatment.
- Prescribers should monitor patients for somnolence and sedation, particularly with concomitant use of other central nervous system depressants.
- Prescribers should caution patients against engaging in hazardous activities requiring mental alertness, such as operating dangerous machinery or motor vehicles, until the effect of ONFI is known.
Potentiation of Sedation from Concomitant Use with Central Nervous System Depressants
- Since ONFI has a central nervous system (CNS) depressant effect, patients or their caregivers should be cautioned against simultaneous use with other CNS depressant drugs or alcohol, and cautioned that the effects of other CNS depressant drugs or alcohol may be potentiated.
Withdrawal Symptoms
- Abrupt discontinuation of ONFI should be avoided.
- ONFI should be tapered by decreasing the dose every week by 5-10 mg/day until discontinuation.
- Withdrawal symptoms occurred following abrupt discontinuation of ONFI; the risk of withdrawal symptoms is greater with higher doses.
- As with all antiepileptic drugs, ONFI should be withdrawn gradually to minimize the risk of precipitating seizures, seizure exacerbation, or status epilepticus.
- Withdrawal symptoms (e.g., convulsions, psychosis, hallucinations, behavioral disorder, tremor, and anxiety) have been reported following abrupt discontinuance of benzodiazepines.
- The more severe withdrawal symptoms have usually been limited to patients who received excessive doses over an extended period of time, followed by an abrupt discontinuation.
- Generally milder withdrawal symptoms (e.g., dysphoria, anxiety, and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic doses for several months.
Serious Dermatological Reactions
- Serious skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with ONFI in both children and adults during the post-marketing period.
- Patients should be closely monitored for signs or symptoms of SJS/TEN, especially during the first 8 weeks of treatment initiation or when re-introducing therapy.
- ONFI should be discontinued at the first sign of rash, unless the rash is clearly not drug-related.
- If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered.
Physical and Psychological Dependence
- Patients with a history of substance abuse should be under careful surveillance when receiving ONFI or other psychotropic agents because of the predisposition of such patients to habituation and dependence.
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including ONFI, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. *Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted relative risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED treated patients was 0.43%, compared to 0.24% among 16,029 placebo treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated.
- There were four suicides in drug treated patients in the trials and none in placebo treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed.
- Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed.
- The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
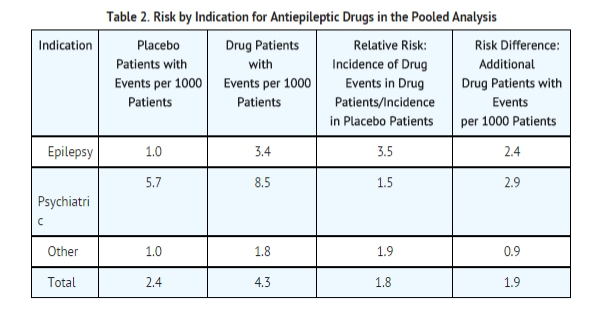
- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
- Anyone considering prescribing ONFI or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness.
- Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior.
- Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm.
- Behaviors of concern should be reported immediately to healthcare providers.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- During its development for the adjunctive treatment of seizures associated with LGS, ONFI was administered to 333 healthy volunteers and 300 patients with a current or prior diagnosis of LGS, including 197 patients treated for 12 months or more.
- The conditions and duration of exposure varied greatly and included single- and multiple-dose clinical pharmacology studies in healthy volunteers and two double-blind studies in patients with LGS (Study 1 and 2). Only Study 1 included a placebo group, allowing comparison of adverse reaction rates on ONFI at several doses to placebo.
Adverse Reactions Leading to Discontinuation in an LGS Placebo Controlled Clinical Trial (Study 1)
- The adverse reactions associated with ONFI treatment discontinuation in ≥1% of patients in decreasing order of frequency included lethargy, somnolence, ataxia, aggression, fatigue, and insomnia.
Most Common Adverse Reactions in an LGS Placebo Controlled Clinical Trial (Study 1) Table 3 lists the adverse reactions that occurred in ≥5% of ONFI treated patients (at any dose), and at a rate greater than placebo treated patients, in the randomized, double-blind, placebo-controlled, parallel group clinical study of adjunctive AED therapy for 15 weeks (Study 1).
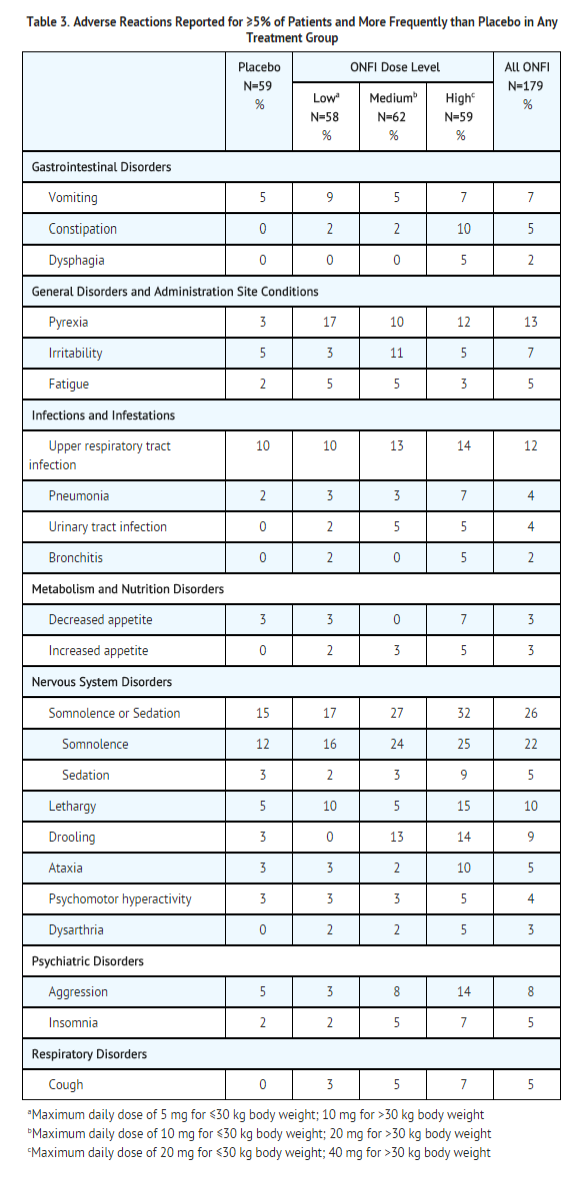
Postmarketing Experience
These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions are categorized by system organ class.
Blood Disorders
- Anemia
- Eosinophilia
- Leukopenia
- Thrombocytopenia
Eye Disorders
- Diplopia
- Vision blurred
Gastrointestinal Disorders
- Abdominal distention
General Disorders and Administration Site Conditions
- Hypothermia
Investigations
- Hepatic enzyme increased
Musculoskeletal
- Muscle spasms
Psychiatric Disorders
- Agitation
- Anxiety
- Apathy
- Confusional state
- Depression
- Delirium
- Delusion
- Hallucination
Renal and Urinary Disorders
- Urinary retention
Respiratory Disorders
- Aspiration
- Respiratory depression
Skin and Subcutaneous Tissue Disorders
- Rash
- Urticaria
- Angioedema
- Facial and lip edema
Drug Interactions
There is limited information regarding Clobazam Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Clobazam in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clobazam in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clobazam during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Clobazam in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Clobazam in pediatric settings.
Geriatic Use
Plasma concentrations at any given dose are generally higher in the elderly: proceed slowly with dose escalation. The starting dose should be 5 mg/day for all elderly patients. Then titrate elderly patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on weight) may be started on day 21 [see Use in Specific Populations (8.5)].
Gender
There is no FDA guidance on the use of Clobazam with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clobazam with respect to specific racial populations.
Renal Impairment
No dose adjustment is required for patients with mild and moderate renal impairment. There is no experience with ONFI in patients with severe renal impairment or end stage renal disease (ESRD). It is not known if clobazam or its active metabolite, N-desmethylclobazam, is dialyzable [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
Hepatic Impairment
ONFI is hepatically metabolized; however, there are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of ONFI. For this reason, proceed slowly with dosing escalations. For patients with mild to moderate hepatic impairment (Child-Pugh score 5-9), the starting dose should be 5 mg/day in both weight groups. Then titrate patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, start an additional titration on day 21 to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group). There is inadequate information about metabolism of ONFI in patients with severe hepatic impairment. Therefore no dosing recommendation in those patients can be given [see Use in Specific Populations (8.8), Clinical Pharmacology (12.3)].
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clobazam in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clobazam in patients who are immunocompromised.
Dosage Adjustments in CYP2C19 Poor Metabolizers
In CYP2C19 poor metabolizers, levels of N-desmethylclobazam, clobazam's active metabolite, will be increased. Therefore, in patients known to be CYP2C19 poor metabolizers, the starting dose should be 5 mg/day and dose titration should proceed slowly according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group) may be started on day 21 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.5)].
Administration and Monitoring
Administration
- Oral
Important Administration Instructions
Instruct patients to read the "Instructions for Use" carefully for complete directions on how to properly dose and administer ONFI oral suspension.
ONFI Tablet Oral Administration ONFI tablets can be taken with or without food. ONFI tablets can be administered whole, broken in half along the score, or crushed and mixed in applesauce.
ONFI Oral Suspension Oral Administration ONFI oral suspension can be taken with or without food [see Clinical Pharmacology (12.3)].
Shake ONFI Oral Suspension well before every administration. When administering the oral suspension, use only the oral dosing syringe provided with the product. Each carton includes two syringes, but only one syringe should be used for dosing. The second oral syringe is reserved as a replacement in case the first syringe is damaged or lost. Insert the provided adapter firmly into the neck of the bottle before first use and keep the adapter in place for the duration of the usage of the bottle. To withdraw the dose, insert the dosing syringe into the adapter and invert the bottle then slowly pull back the plunger to prescribed dose. After removing the syringe from the bottle adapter, slowly squirt ONFI Oral Suspension into the corner of the patient's mouth. Replace the cap after each use. The cap fits over the adapter when the adapter is properly placed. See ONFI Oral Suspension "Instructions for Use" for complete instruction on how to properly dose and administer the ONFI Oral Suspension.
Monitoring
There is limited information regarding Clobazam Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Clobazam and IV administrations.
Overdosage
There is limited information regarding Clobazam overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Clobazam Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Clobazam Mechanism of Action in the drug label.
Structure
There is limited information regarding Clobazam Structure in the drug label.
Pharmacodynamics
There is limited information regarding Clobazam Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Clobazam Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Clobazam Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Clobazam Clinical Studies in the drug label.
How Supplied
There is limited information regarding Clobazam How Supplied in the drug label.
Storage
There is limited information regarding Clobazam Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Clobazam |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clobazam |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clobazam Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Clobazam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Clobazam Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Clobazam Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.