Cladribine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
|drugClass=[[antineoplastic agent]] | |drugClass=[[antineoplastic agent]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication= active Hairy Cell Leukemia | |indication=active [[Hairy Cell Leukemia]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[Neutropenia]], [[fever]], [[infections]] | |adverseReactions=[[Neutropenia]], [[fever]], [[infections]] | ||
| Line 13: | Line 13: | ||
Acute nephrotoxicity has been observed with high doses of Cladribine (4 to 9 times the recommended dose for Hairy Cell Leukemia), especially when given concomitantly with other nephrotoxic agents/therapies. | Acute nephrotoxicity has been observed with high doses of Cladribine (4 to 9 times the recommended dose for Hairy Cell Leukemia), especially when given concomitantly with other nephrotoxic agents/therapies. | ||
|fdaLIADAdult=====Active Hairy Cell Leukemia==== | |fdaLIADAdult=====Active Hairy Cell Leukemia==== | ||
*Defined by clinically significant anemia, neutropenia, thrombocytopenia or disease-related symptoms | *Defined by clinically significant [[anemia]], [[neutropenia]], [[thrombocytopenia]] or disease-related symptoms | ||
*Dosage: single course given by continuous infusion for 7 consecutive days at a dose of 0.09 mg/kg/day. | *Dosage: single course given by continuous infusion for 7 consecutive days at a dose of 0.09 mg/kg/day. | ||
*If the patient does not respond to the initial course of Cladribine Injection for Hairy Cell Leukemia, it is unlikely that they will benefit from additional courses. | *If the patient does not respond to the initial course of Cladribine Injection for [[Hairy Cell Leukemia]], it is unlikely that they will benefit from additional courses. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Cladribine in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Cladribine in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Cladribine in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Cladribine in adult patients. | ||
| Line 21: | Line 21: | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Cladribine in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Cladribine in pediatric patients. | ||
|contraindications=*Cladribine Injection is contraindicated in those patients who are hypersensitive to this drug or any of its components. | |contraindications=*Cladribine Injection is contraindicated in those patients who are hypersensitive to this drug or any of its components. | ||
|warnings=Severe bone marrow suppression, including neutropenia, anemia and thrombocytopenia, has been commonly observed in patients treated with Cladribine, especially at high doses. At initiation of treatment, most patients in the clinical studies had hematologic impairment as a manifestation of active Hairy Cell Leukemia. Following treatment with Cladribine, further hematologic impairment occurred before recovery of peripheral blood counts began. During the first two weeks after treatment initiation, mean Platelet Count, ANC, and Hemoglobin concentration declined and subsequently increased with normalization of mean counts by Day 12, Week 5 and Week 8, respectively. The myelosuppressive effects of Cladribine were most notable during the first month following treatment. Forty-four percent (44%) of patients received transfusions with | |warnings=*'''Severe [[bone marrow suppression]]''', including [[neutropenia]], [[anemia]] and [[thrombocytopenia]], has been commonly observed in patients treated with Cladribine, especially at high doses. | ||
*At initiation of treatment, most patients in the clinical studies had hematologic impairment as a manifestation of active [[Hairy Cell Leukemia]]. | |||
*Following treatment with Cladribine, further hematologic impairment occurred before recovery of peripheral blood counts began. | |||
*During the first two weeks after treatment initiation, mean Platelet Count, ANC, and Hemoglobin concentration declined and subsequently increased with normalization of mean counts by Day 12, Week 5 and Week 8, respectively. | |||
*The myelosuppressive effects of Cladribine were most notable during the first month following treatment. | |||
*Forty-four percent (44%) of patients received transfusions with [[RBC]]s and 14% received transfusions with platelets during Month 1. | |||
*Careful hematologic monitoring, especially during the first 4 to 8 weeks after treatment with Cladribine Injection, is recommended. | |||
Fever (T ≥ 100°F) was associated with the use of Cladribine in approximately two-thirds of patients (131/196) in the first month of therapy. Virtually all of these patients were treated empirically with parenteral antibiotics. Overall, 47% (93/196) of all patients had fever in the setting of neutropenia (ANC ≤ 1000), including 62 patients (32%) with severe neutropenia (i.e., ANC ≤ 500). | *'''Fever''' (T ≥ 100°F) was associated with the use of Cladribine in approximately two-thirds of patients (131/196) in the first month of therapy. | ||
*Virtually all of these patients were treated empirically with parenteral antibiotics. Overall, 47% (93/196) of all patients had fever in the setting of [[neutropenia]] (ANC ≤ 1000), including 62 patients (32%) with severe [[neutropenia]] (i.e., ANC ≤ 500). | |||
*In a Phase I investigational study using Cladribine in high doses (4 to 9 times the recommended dose for [[Hairy Cell Leukemia]]) as part of a [[bone marrow transplant]] conditioning regimen, which also included high dose [[cyclophosphamide]] and total body irradiation, acute [[nephrotoxicity]] and delayed onset [[neurotoxicity]] were observed. | |||
*Thirty one (31) poor-risk patients with drug-resistant acute [[leukemia]] in relapse (29 cases) or [[non-Hodgkins Lymphoma]] (2 cases) received Cladribine for 7 to 14 days prior to bone marrow transplantation. | |||
*During infusion, 8 patients experienced gastrointestinal symptoms. | |||
*While the [[bone marrow]] was initially cleared of all hematopoietic elements, including tumor cells, [[leukemia]] eventually recurred in all treated patients. Within 7 to 13 days after starting treatment with Cladribine, 6 patients (19%) developed manifestations of renal dysfunction (e.g., [[acidosis]], [[anuria]], elevated serum [[creatinine]], etc.) and 5 required dialysis. | |||
*Several of these patients were also being treated with other medications having known nephrotoxic potential. | |||
*[[Renal dysfunction]] was reversible in 2 of these patients. In the 4 patients whose renal function had not recovered at the time of death, autopsies were performed; in 2 of these, evidence of tubular damage was noted. | |||
*Eleven (11) patients (35%) experienced delayed onset [[neurologic toxicity]]. | |||
*In the majority, this was characterized by progressive irreversible motor weakness ([[paraparesis]]/[[quadriparesis]]) of the upper and/or lower extremities, first noted 35 to 84 days after starting high dose therapy with Cladribine. | |||
*Non-invasive testing (electromyography and nerve conduction studies) was consistent with [[demyelinating disease]]. Severe [[neurologic toxicity]] has also been noted with high doses of another drug in this class. | |||
[[Axonal peripheral polyneuropathy]] was observed in a dose escalation study at the highest dose levels (approximately 4 times the recommended dose for [[Hairy Cell Leukemia]]) in patients not receiving [[cyclophosphamide]] or total body irradiation. | |||
*Severe neurological toxicity has been reported rarely following treatment with standard cladribine dosing regimens. | |||
In patients with [[Hairy Cell Leukemia]] treated with the recommended treatment regimen (0.09 mg/kg/day for 7 consecutive days), there have been no reports of nephrologic toxicities. | |||
*Of the 196 [[Hairy Cell Leukemia]] patients entered in the two trials, there were 8 deaths following treatment. Of these, 6 were of infectious etiology, including 3 pneumonias, and 2 occurred in the first month following Cladribine therapy. Of the 8 deaths, 6 occurred in previously treated patients who were refractory to [[α interferon]]. | |||
*'''[[Benzyl alcohol]]''' is a constituent of the recommended diluent for the 7-day infusion solution. Benzyl alcohol has been reported to be associated with a fatal '''"[[Gasping Syndrome]]"''' in premature infants. | |||
|clinicalTrials=''Safety data are based on 196 patients with [[Hairy Cell Leukemia]]:'' the original cohort of 124 patients plus an additional 72 patients enrolled at the same two centers after the original enrollment cutoff. | |||
*In Month 1 of the [[Hairy Cell Leukemia]] [[clinical trials]], severe [[neutropenia]] was noted in 70% of patients, [[fever]] in 69%, and [[infection]] was documented in 28%. Other adverse experiences reported frequently during the first 14 days after initiating treatment included: [[fatigue]] (45%), [[nausea]] (28%), [[rash]] (27%), [[headache]] (22%) and injection site reactions (19%). Most non-hematologic adverse experiences were mild to moderate in severity. | |||
[[Myelosuppression]] was frequently observed during the first month after starting treatment. [[Neutropenia]] (ANC < 500 x 106/L) was noted in 70% of patients, compared with 26% in whom it was present initially. Severe [[anemia]] ([[Hemoglobin]] < 8.5 g/dL) developed in 37% of patients, compared with 10% initially and [[thrombocytopenia]] ([[Platelets]] < 20 x 109/L) developed in 12% of patients, compared to 4% in whom it was noted initially. During the first month, 54 of 196 patients (28%) exhibited documented evidence of infection. | |||
Serious infections (e.g., [[septicemia]], [[pneumonia]]) were reported in 6% of all patients; the remainder were mild or moderate. Several deaths were attributable to infection and/or complications related to the underlying disease. | |||
During the second month, the overall rate of documented infection was 6%; these infections were mild to moderate and no severe systemic infections were seen. After the third month, the monthly incidence of infection was either less than or equal to that of the months immediately preceding Cladribine therapy. During the first month, 11% of patients experienced severe fever (i.e., ≥104°F). Documented infections were noted in fewer than one-third of febrile episodes. Of the 196 patients studied, 19 were noted to have a documented infection in the month prior to treatment. In the month following treatment, there were 54 episodes of documented infection: 23 (42%) were [[bacterial]], 11 (20%) were [[viral]] and 11 (20%) were [[fungal]]. Seven (7) of 8 documented episodes of [[herpes zoster]] occurred during the month following treatment. Fourteen (14) of 16 episodes of documented fungal infections occurred in the first two months following treatment. Virtually all of these patients were treated empirically with [[antibiotics]]. | |||
Analysis of [[lymphocyte]] subsets indicates that treatment with cladribine is associated with prolonged depression of the [[CD4]] counts. Prior to treatment, the mean [[CD4]] count was 766/μL. The mean [[CD4]] count nadir, which occurred 4 to 6 months following treatment, was 272/μL. Fifteen (15) months after treatment, mean [[CD4]] counts remained below 500/μL. CD8 counts behaved similarly, though increasing counts were observed after 9 months. The clinical significance of the prolonged [[CD4]] [[lymphopenia]] is unclear. | |||
Another event of unknown clinical significance includes the observation of prolonged [[bone marrow]] hypocellularity. [[Bone marrow]] cellularity of < 35% was noted after 4 months in 42 of 124 patients (34%) treated in the two pivotal trials. This hypocellularity was noted as late as day 1010. It is not known whether the hypocellularity is the result of disease related marrow fibrosis or if it is the result of cladribine toxicity. There was no apparent clinical effect on the peripheral blood counts. | |||
The vast majority of rashes were mild and occurred in patients who were receiving or had recently been treated with other medications (e.g., [[allopurinol]] or [[antibiotics]]) known to cause [[rash]]. | |||
Most episodes of [[nausea]] were mild, not accompanied by [[vomiting]], and did not require treatment with [[antiemetics]]. In patients requiring [[antiemetics]], [[nausea]] was easily controlled, most frequently with chlorpromazine. | |||
Most episodes of nausea were mild, not accompanied by vomiting, and did not require treatment with antiemetics. In patients requiring antiemetics, nausea was easily controlled, most frequently with chlorpromazine. | |||
Adverse reactions reported during the first 2 weeks following treatment initiation (regardless of relationship to drug) by > 5% of patients included: | Adverse reactions reported during the first 2 weeks following treatment initiation (regardless of relationship to drug) by > 5% of patients included: | ||
Body as a Whole | ====Body as a Whole==== | ||
*[[Fever]] (69%) | |||
*[[Fatigue]] (45%), | |||
*[[Chills]] (9%) | |||
*[[Asthenia]] (9%) | |||
*[[Diaphoresis]] (9%) | |||
*[[Malaise]] (7%) | |||
*Trunk [[pain]] (6%) | |||
( | ====Gastrointestinal==== | ||
*[[Nausea]] (28%) | |||
*[[Decreased appetite]] (17%) | |||
*[[Vomiting]] (13%) | |||
*[[Diarrhea]] (10%) | |||
*[[Constipation]] (9%) | |||
*[[Abdominal pain]] (6%) | |||
====Hemic/Lymphatic==== | |||
*[[Purpura]] (10%) | |||
*[[Petechiae]] (8%) | |||
*[[Epistaxis]] (5%) | |||
( | ====Nervous System==== | ||
*[[Headache]] (22%) | |||
*[[Dizziness]] (9%) | |||
*[[Insomnia]] (7%) | |||
====Cardiovascular System==== | |||
*[[Edema]] (6%) | |||
*[[Tachycardia]] (6%) | |||
====Respiratory System==== | |||
*Abnormal breath sounds (11%) | |||
*[[Cough]] (10%) | |||
*Abnormal chest sounds (9%) | |||
*Shortness of breath (7%) | |||
====Skin/Subcutaneous Tissue==== | |||
*[[Rash]] (27%) | |||
*Injection site reactions (19%) | |||
*[[Pruritis]] (6%) | |||
*[[Pain]] (6%) | |||
*[[Erythema]] (6%) | |||
====Musculoskeletal System==== | |||
*[[Myalgia]] (7%) | |||
*[[Arthralgia]] (5%) | |||
====Adverse experiences related to intravenous administration included==== | |||
*Injection site reactions (9%) (i.e., redness, swelling, pain), | |||
*Thrombosis (2%) | |||
*Phlebitis (2%) | |||
*Broken catheter (1%). | |||
These appear to be related to the infusion procedure and/or indwelling catheter, rather than the medication or the vehicle. | |||
From Day 15 to the last follow-up visit, the only events reported by > 5% of patients were: [[fatigue]] (11%), [[rash]] (10%), [[headache]] (7%), [[cough]] (7%), and [[malaise]] (5%). | |||
For a description of adverse reactions associated with use of high doses in non-[[Hairy Cell Leukemia]] patients. | |||
|postmarketing=The following additional adverse events have been reported since the drug became commercially available. These adverse events have been reported primarily in patients who received multiple courses of Cladribine Injection: | |||
====Hematologic==== | |||
*[[Bone marrow suppression]] with prolonged [[pancytopenia]], including some reports of [[aplastic anemia]]; [[hemolytic anemia]], which was reported in patients with lymphoid malignancies, occurring within the first few weeks following treatment. Rare cases of [[myelodysplastic syndrome]] have been reported. | |||
====Hepatic==== | |||
*Reversible, generally mild increases in [[bilirubin]] and [[transaminases]]. | |||
====Nervous System==== | |||
*[[Neurological toxicity]]; however, severe neurotoxicity has been reported rarely following treatment with standard cladribine dosing regimens. | |||
====Respiratory System==== | |||
*Pulmonary interstitial infiltrates; in most cases, an infectious etiology was identified. | |||
====Skin/Subcutaneous==== | |||
*[[Urticaria]] | |||
*[[Hypereosinophilia]] | |||
*In isolated cases [[Stevens-Johnson]] and [[toxic epidermal necrolysis]] have been reported in patients who were receiving or had recently been treated with other medications (e.g., [[allopurinol]] or [[antibiotics]]) known to cause these syndromes. | |||
[[Opportunistic infections]] have occurred in the acute phase of treatment due to the [[immunosuppression]] mediated by Cladribine Injection. | |||
|drugInteractions=There are no known drug interactions with Cladribine Injection. Caution should be exercised if Cladribine Injection is administered before, after, or in conjunction with other drugs known to cause [[immunosuppression]] or [[myelosuppression]]. | |||
Opportunistic infections have occurred in the acute phase of treatment due to the immunosuppression mediated by Cladribine Injection. | |||
|drugInteractions=There are no known drug interactions with Cladribine Injection. Caution should be exercised if Cladribine Injection is administered before, after, or in conjunction with other drugs known to cause immunosuppression or myelosuppression. | |||
|FDAPregCat=D | |FDAPregCat=D | ||
|useInPregnancyFDA=Cladribine Injection should not be given during pregnancy. | |useInPregnancyFDA=*Cladribine Injection should not be given during pregnancy. | ||
*Cladribine is teratogenic in mice and rabbits and consequently has the potential to cause fetal harm when administered to a pregnant woman. | |||
Cladribine is teratogenic in mice and rabbits and consequently has the potential to cause fetal harm when administered to a pregnant woman. A significant increase in fetal variations was observed in mice receiving 1.5 mg/kg/day (4.5 mg/m2) and increased resorptions, reduced litter size and increased fetal malformations were observed when mice received 3.0 mg/kg/day (9 mg/m2). Fetal death and malformations were observed in rabbits that received 3.0 mg/kg/day (33.0 mg/m2). No fetal effects were seen in mice at 0.5 mg/kg/day (1.5 mg/m2) or in rabbits at 1.0 mg/kg/day (11.0 mg/m2). | *A significant increase in fetal variations was observed in mice receiving 1.5 mg/kg/day (4.5 mg/m2) and increased resorptions, reduced litter size and increased fetal malformations were observed when mice received 3.0 mg/kg/day (9 mg/m2). Fetal death and malformations were observed in rabbits that received 3.0 mg/kg/day (33.0 mg/m2). | ||
*No fetal effects were seen in mice at 0.5 mg/kg/day (1.5 mg/m2) or in rabbits at 1.0 mg/kg/day (11.0 mg/m2). | |||
Although there is no evidence of teratogenicity in humans due to Cladribine, other drugs which inhibit DNA synthesis (e.g., methotrexate and aminopterin) have been reported to be teratogenic in humans. Cladribine has been shown to be embryotoxic in mice when given at doses equivalent to the recommended dose. | *Although there is no evidence of teratogenicity in humans due to Cladribine, other drugs which inhibit DNA synthesis (e.g., [[methotrexate]] and [[aminopterin]]) have been reported to be teratogenic in humans. | ||
*Cladribine has been shown to be embryotoxic in mice when given at doses equivalent to the recommended dose. | |||
There are no adequate and well controlled studies in pregnant women. If Cladribine is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing age should be advised to avoid becoming pregnant. | *There are no adequate and well controlled studies in pregnant women. If Cladribine is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing age should be advised to avoid becoming pregnant. | ||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from cladribine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother. | |useInNursing=*It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from cladribine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother. | ||
|useInPed=Safety and effectiveness in pediatric patients have not been established. In a Phase I study involving patients 1-21 years old with relapsed acute leukemia, Cladribine was given by continuous intravenous infusion in doses ranging from 3 to 10.7 mg/m2/day for 5 days (one-half to twice the dose recommended in Hairy Cell Leukemia). In this study, the dose-limiting toxicity was severe myelosuppression with profound neutropenia and thrombocytopenia. At the highest dose (10.7 mg/m2/day), 3 of 7 patients developed irreversible myelosuppression and fatal systemic bacterial or fungal infections. No unique toxicities were noted in this study | |useInPed=*Safety and effectiveness in pediatric patients have not been established. | ||
|useInGeri=Clinical studies of Cladribine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients. | *In a Phase I study involving patients 1-21 years old with relapsed [[acute leukemia]], Cladribine was given by continuous intravenous infusion in doses ranging from 3 to 10.7 mg/m2/day for 5 days (one-half to twice the dose recommended in [[Hairy Cell Leukemia]]). | ||
*In this study, the dose-limiting toxicity was severe [[myelosuppression]] with profound [[neutropenia]] and [[thrombocytopenia]]. | |||
*At the highest dose (10.7 mg/m2/day), 3 of 7 patients developed irreversible myelosuppression and fatal systemic bacterial or fungal infections. No unique toxicities were noted in this study | |||
|useInGeri=*Clinical studies of Cladribine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. | |||
*In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients. | |||
|useInRenalImpair=*There are inadequate data on dosing of patients with renal insufficiency. | |useInRenalImpair=*There are inadequate data on dosing of patients with renal insufficiency. | ||
*Development of acute renal insufficiency in some patients receiving high doses of Cladribine has been described. | *Development of acute renal insufficiency in some patients receiving high doses of Cladribine has been described. | ||
|useInHepaticImpair=*There are inadequate data on dosing of patients with hepatic insufficiency. | |useInHepaticImpair=*There are inadequate data on dosing of patients with hepatic insufficiency. | ||
|administration=*Intravenous Infusion | |administration=*Intravenous Infusion | ||
|monitoring=*Periodic assessment of peripheral blood counts, particularly during the first 4 to 8 weeks post-treatment, is recommended to detect the development of anemia, neutropenia and thrombocytopenia and for early detection of any potential sequelae (e.g., infection or bleeding). *As with other potent chemotherapeutic agents, monitoring of renal and hepatic function is also recommended, especially in patients with underlying kidney or liver dysfunction. | |monitoring=*Periodic assessment of peripheral blood counts, particularly during the first 4 to 8 weeks post-treatment, is recommended to detect the development of [[anemia]], [[neutropenia]] and [[thrombocytopenia]] and for early detection of any potential sequelae (e.g., infection or bleeding). *As with other potent [[chemotherapeutic agents]], monitoring of renal and hepatic function is also recommended, especially in patients with underlying kidney or liver dysfunction. | ||
*Fever was a frequently observed side effect during the first month on study. Since the majority of fevers occurred in neutropenic patients, patients should be closely monitored during the first month of treatment and empiric antibiotics should be initiated as clinically indicated. | *Fever was a frequently observed side effect during the first month on study. Since the majority of fevers occurred in neutropenic patients, patients should be closely monitored during the first month of treatment and empiric [[antibiotics]] should be initiated as clinically indicated. | ||
|IVCompat=Preparation and Administration of Intravenous Solutions: | |IVCompat='''Preparation and Administration of Intravenous Solutions:'''<br> | ||
*Cladribine Injection must be diluted with the designated diluent prior to administration. | |||
Cladribine Injection must be diluted with the designated diluent prior to administration. Since the drug product does not contain any anti-microbial preservative or bacteriostatic agent, aseptic technique and proper environmental precautions must be observed in preparation of Cladribine Injection solutions. | *Since the drug product does not contain any anti-microbial preservative or bacteriostatic agent, aseptic technique and proper environmental precautions must be observed in preparation of Cladribine Injection solutions. | ||
Add the calculated dose (0.09 mg/kg or 0.09 mL/kg) of Cladribine Injection to an infusion bag containing 500 mL of 0.9% Sodium Chloride Injection, USP. Infuse continuously over 24 hours. Repeat daily for a total of 7 consecutive days. The use of 5% dextrose as a diluent is not recommended because of increased degradation of cladribine. Admixtures of Cladribine Injection are chemically and physically stable for at least 24 hours at room temperature under normal room fluorescent light in | '''To prepare a single daily dose:''' | ||
*Add the calculated dose (0.09 mg/kg or 0.09 mL/kg) of Cladribine Injection to an infusion bag containing 500 mL of 0.9% Sodium Chloride Injection, USP. | |||
*Infuse continuously over 24 hours. | |||
*Repeat daily for a total of 7 consecutive days. | |||
*The use of 5% dextrose as a diluent is not recommended because of increased degradation of cladribine. | |||
*Admixtures of Cladribine Injection are chemically and physically stable for at least 24 hours at room temperature under normal room fluorescent light in PVC infusion containers. | |||
*Since limited compatibility data are available, adherence to the recommended diluents and infusion systems is advised. | |||
[[File:Cladribine IV.png|none|400px]] | [[File:Cladribine IV.png|none|400px]] | ||
To prepare a 7-day infusion: | '''To prepare a 7-day infusion:'''<br> | ||
*The 7-day infusion solution should only be prepared with [[Bacteriostatic]] 0.9% [[Sodium Chloride]] Injection, USP (0.9% [[benzyl alcohol]] preserved). | |||
The 7-day infusion solution should only be prepared with Bacteriostatic 0.9% Sodium Chloride Injection, USP (0.9% benzyl alcohol preserved). In order to minimize the risk of microbial contamination, both Cladribine Injection and the diluent should be passed through a sterile 0.22μ disposable hydrophilic syringe filter as each solution is being introduced into the infusion reservoir. First add the calculated dose of Cladribine Injection (7 days x 0.09 mg/kg or mL/kg) to the infusion reservoir through the sterile filter. | *In order to minimize the risk of microbial contamination, both Cladribine Injection and the diluent should be passed through a sterile 0.22μ disposable hydrophilic syringe filter as each solution is being introduced into the infusion reservoir. | ||
*First add the calculated dose of Cladribine Injection (7 days x 0.09 mg/kg or mL/kg) to the infusion reservoir through the sterile filter. | |||
Then add a calculated amount of Bacteriostatic 0.9% Sodium Chloride Injection, USP (0.9% benzyl alcohol preserved) also through the filter to bring the total volume of the solution to 100 mL. After completing solution preparation, clamp off the line, disconnect and discard the filter. Aseptically aspirate air bubbles from the reservoir as necessary using the syringe and a dry second sterile filter or a sterile vent filter assembly. Reclamp the line and discard the syringe and filter assembly. Infuse continuously over 7 days. Solutions prepared with Bacteriostatic Sodium Chloride Injection for individuals weighing more than 85 kg may have reduced preservative effectiveness due to greater dilution of the benzyl alcohol preservative. Admixtures for the 7-day infusion have demonstrated acceptable chemical and physical stability for at least 7 days in the MEDICATION CASSETTE Reservoir. | *Then add a calculated amount of [[Bacteriostatic]] 0.9% Sodium Chloride Injection, USP (0.9% [[benzyl alcohol]] preserved) also through the filter to bring the total volume of the solution to 100 mL. | ||
*After completing solution preparation, clamp off the line, disconnect and discard the filter. *Aseptically aspirate air bubbles from the reservoir as necessary using the syringe and a dry second sterile filter or a sterile vent filter assembly. | |||
*Reclamp the line and discard the syringe and filter assembly. | |||
*Infuse continuously over 7 days. | |||
*Solutions prepared with [[Bacteriostatic]] [[Sodium Chloride]] Injection for individuals weighing more than 85 kg may have reduced preservative effectiveness due to greater dilution of the [[benzyl alcohol]] preservative. | |||
*Admixtures for the 7-day infusion have demonstrated acceptable chemical and physical stability for at least 7 days in the MEDICATION CASSETTE Reservoir. | |||
[[File:Cladribine IV 2.png|none|400px]] | [[File:Cladribine IV 2.png|none|400px]] | ||
Since limited compatibility data are available, adherence to the recommended diluents and infusion systems is advised. Solutions containing Cladribine Injection should not be mixed with other intravenous drugs or additives or infused simultaneously via a common intravenous line, since compatibility testing has not been performed. Preparations containing benzyl alcohol should not be used in neonates ( | *Since limited compatibility data are available, adherence to the recommended diluents and infusion systems is advised. | ||
*Solutions containing Cladribine Injection should not be mixed with other intravenous drugs or additives or infused simultaneously via a common intravenous line, since compatibility testing has not been performed. | |||
*Preparations containing [[benzyl alcohol]] should not be used in neonates. | |||
*Care must be taken to assure the sterility of prepared solutions. | |||
*Once diluted, solutions of Cladribine Injection should be administered promptly or stored in the refrigerator (2° to 8° C) for no more than 8 hours prior to start of administration. | |||
*Vials of Cladribine Injection are for single-use only. Any unused portion should be discarded in an appropriate manner | |||
*Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. | |||
*A precipitate may occur during the exposure of Cladribine Injection to low temperatures; it may be resolubilized by allowing the solution to warm naturally to room temperature and by shaking vigorously. | |||
*DO NOT HEAT OR MICROWAVE. | |||
'''Chemical Stability of Vials:''' | |||
*When stored in refrigerated conditions between 2° to 8°C (36° to 46°F) protected from light, unopened vials of Cladribine Injection are stable until the expiration date indicated on the package. | |||
*Freezing does not adversely affect the solution. | |||
*If freezing occurs, thaw naturally to room temperature. | |||
*DO NOT heat or microwave. | |||
*Once thawed, the vial of Cladribine Injection is stable until expiry if refrigerated. | |||
*DO NOT refreeze. | |||
*Once diluted, solutions containing Cladribine Injection should be administered promptly or stored in the refrigerator (2° to 8°C) for no more than 8 hours prior to administration. | |||
'''Handling and Disposal:''' | |||
*The potential hazards associated with [[cytotoxic]] agents are well established and proper precautions should be taken when handling, preparing, and administering Cladribine Injection. *The use of disposable gloves and protective garments is recommended. | |||
*If Cladribine Injection contacts the skin or mucous membranes, wash the involved surface immediately with copious amounts of water. | |||
Handling and Disposal: | *Several guidelines on this subject have been published.(2-8) There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. Refer to your Institution's guidelines and all applicable state/local regulations for disposal of cytotoxic waste. | ||
The potential hazards associated with cytotoxic agents are well established and proper precautions should be taken when handling, preparing, and administering Cladribine Injection. The use of disposable gloves and protective garments is recommended. If Cladribine Injection contacts the skin or mucous membranes, wash the involved surface immediately with copious amounts of water. Several guidelines on this subject have been published.(2-8) There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. Refer to your Institution's guidelines and all applicable state/local regulations for disposal of cytotoxic waste. | |overdose='''High doses of Cladribine have been associated with:''' | ||
|overdose=High doses of Cladribine have been associated with: | *Irreversible neurologic toxicity ([[paraparesis]]/[[quadriparesis]]), [[acute nephrotoxicity]], and [[severe bone marrow suppression]] resulting in [[neutropenia]], [[anemia]] and [[thrombocytopenia]]. | ||
*There is no known specific antidote to overdosage. | |||
*Treatment of overdosage consists of discontinuation of Cladribine, careful observation and appropriate supportive measures. | |||
*It is not known whether the drug can be removed from the circulation by dialysis or hemofiltration. | |||
|drugBox={{Drugbox | |drugBox={{Drugbox | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 199: | Line 278: | ||
| StdInChIKey = PTOAARAWEBMLNO-KVQBGUIXSA-N | | StdInChIKey = PTOAARAWEBMLNO-KVQBGUIXSA-N | ||
}} | }} | ||
|mechAction=The selective toxicity of 2-chloro-2΄-deoxy-β-D-adenosine towards certain normal and malignant lymphocyte and monocyte populations is based on the relative activities of deoxycytidine kinase and deoxynucleotidase. Cladribine passively crosses the cell membrane. In cells with a high ratio of deoxycytidine kinase to deoxynucleotidase, it is phosphorylated by deoxycytidine kinase to 2-chloro-2΄-deoxy- β -D-adenosine monophosphate (2-CdAMP). Since 2-chloro-2΄-deoxy- β -D-adenosine is resistant to deamination by adenosine deaminase and there is little deoxynucleotide deaminase in lymphocytes and monocytes, 2-CdAMP accumulates intracellularly and is subsequently converted into the active triphosphate deoxynucleotide, 2-chloro-2΄-deoxy- β -D-adenosine triphosphate (2-CdATP). It is postulated that cells with high deoxycytidine kinase and low deoxynucleotidase activities will be selectively killed by 2-chloro-2΄-deoxy- β -D-adenosine as toxic deoxynucleotides accumulate intracellularly. | |mechAction=*The selective toxicity of 2-chloro-2΄-deoxy-β-D-adenosine towards certain normal and malignant [[lymphocyte]] and [[monocyte]] populations is based on the relative activities of [[deoxycytidine kinase]] and deoxynucleotidase. | ||
*Cladribine passively crosses the [[cell membrane]]. | |||
Cells containing high concentrations of deoxynucleotides are unable to properly repair single-strand DNA breaks. The broken ends of DNA activate the enzyme poly (ADP-ribose) polymerase resulting in NAD and ATP depletion and disruption of cellular metabolism. There is evidence, also, that 2-CdATP is incorporated into the DNA of dividing cells, resulting in impairment of DNA synthesis. Thus, 2-chloro-2΄-deoxy- β -D-adenosine can be distinguished from other chemotherapeutic agents affecting purine metabolism in that it is cytotoxic to both actively dividing and quiescent lymphocytes and monocytes, inhibiting both DNA synthesis and repair. | *In cells with a high ratio of [[deoxycytidine kinase]] to deoxynucleotidase, it is phosphorylated by deoxycytidine kinase to 2-chloro-2΄-deoxy- β -D-adenosine monophosphate (2-CdAMP). *Since 2-chloro-2΄-deoxy- β -D-adenosine is resistant to deamination by [[adenosine deaminase]] and there is little [[deoxynucleotide deaminase]] in [[lymphocytes]] and [[monocytes]], 2-CdAMP accumulates intracellularly and is subsequently converted into the active triphosphate deoxynucleotide, 2-chloro-2΄-deoxy- β -D-adenosine triphosphate (2-CdATP). | ||
|structure=Cladribine Injection (also commonly known as 2-chloro-2΄-deoxy- β -D-adenosine) is a synthetic antineoplastic agent for continuous intravenous infusion. It is a clear, colorless, sterile, preservative-free, isotonic solution. Cladribine Injection is available in single-use vials containing 10 mg (1 mg/mL) of cladribine, a chlorinated purine nucleoside analog. Each milliliter of Cladribine Injection contains 1 mg of the active ingredient and 9 mg (0.15 mEq) of sodium chloride as an inactive ingredient. The solution has a pH range of 5.5 to 8.0. Phosphoric acid and/or dibasic sodium phosphate may have been added to adjust the pH to 6.3±0.3. | *It is postulated that cells with high deoxycytidine kinase and low deoxynucleotidase activities will be selectively killed by 2-chloro-2΄-deoxy- β -D-adenosine as toxic deoxynucleotides accumulate intracellularly. | ||
*Cells containing high concentrations of deoxynucleotides are unable to properly repair single-strand [[DNA]] breaks. The broken ends of [[DNA]] activate the enzyme poly (ADP-ribose) polymerase resulting in [[NAD]] and [[ATP]] depletion and disruption of cellular metabolism. | |||
*There is evidence, also, that 2-CdATP is incorporated into the [[DNA]] of dividing cells, resulting in impairment of [[DNA synthesis]]. | |||
*Thus, 2-chloro-2΄-deoxy- β -D-adenosine can be distinguished from other chemotherapeutic agents affecting [[purine metabolism]] in that it is [[cytotoxic]] to both actively dividing and quiescent [[lymphocytes]] and [[monocytes]], inhibiting both [[DNA synthesis]] and repair. | |||
|structure=Cladribine Injection (also commonly known as 2-chloro-2΄-deoxy- β -D-adenosine) is a synthetic [[antineoplastic agent]] for continuous intravenous infusion. It is a clear, colorless, sterile, preservative-free, isotonic solution. Cladribine Injection is available in single-use vials containing 10 mg (1 mg/mL) of cladribine, a chlorinated purine nucleoside analog. Each milliliter of Cladribine Injection contains 1 mg of the active ingredient and 9 mg (0.15 mEq) of sodium chloride as an inactive ingredient. The solution has a pH range of 5.5 to 8.0. Phosphoric acid and/or dibasic sodium phosphate may have been added to adjust the pH to 6.3±0.3. | |||
The chemical name for cladribine is 2-chloro-6-amino-9-(2-deoxy-β-D-erythropento-furanosyl) purine and the structure is represented below: | The chemical name for cladribine is 2-chloro-6-amino-9-(2-deoxy-β-D-erythropento-furanosyl) purine and the structure is represented below: | ||
[[File:Cladribine Chemical structure.png|none|400px]] | [[File:Cladribine Chemical structure.png|none|400px]] | ||
|PK=In a clinical investigation, 17 patients with Hairy Cell Leukemia and normal renal function were treated for 7 days with the recommended treatment regimen of Cladribine Injection (0.09 mg/kg/day) by continuous intravenous infusion. The mean steady-state serum concentration was estimated to be 5.7 ng/mL with an estimated systemic clearance of 663.5 mL/h/kg when Cladribine was given by continuous infusion over 7 days. In Hairy Cell Leukemia patients, there does not appear to be a relationship between serum concentrations and ultimate clinical outcome. | |PK=In a clinical investigation, 17 patients with [[Hairy Cell Leukemia]] and normal [[renal function]] were treated for 7 days with the recommended treatment regimen of Cladribine Injection (0.09 mg/kg/day) by continuous intravenous infusion. The mean steady-state serum concentration was estimated to be 5.7 ng/mL with an estimated systemic clearance of 663.5 mL/h/kg when Cladribine was given by continuous infusion over 7 days. In [[Hairy Cell Leukemia]] patients, there does not appear to be a relationship between serum concentrations and ultimate clinical outcome. | ||
In another study, 8 patients with hematologic malignancies received a two (2) hour infusion of Cladribine Injection (0.12 mg/kg). The mean end-of-infusion plasma Cladribine concentration was 48±19 ng/mL. For 5 of these patients, the disappearance of Cladribine could be described by either a biphasic or triphasic decline. For these patients with normal renal function, the mean terminal half-life was 5.4 hours. Mean values for clearance and steady-state volume of distribution were 978±422 mL/h/kg and 4.5±2.8 L/kg, respectively. | In another study, 8 patients with hematologic malignancies received a two (2) hour infusion of Cladribine Injection (0.12 mg/kg). The mean end-of-infusion plasma Cladribine concentration was 48±19 ng/mL. For 5 of these patients, the disappearance of Cladribine could be described by either a biphasic or triphasic decline. For these patients with normal [[renal function]], the mean terminal half-life was 5.4 hours. Mean values for clearance and steady-state volume of distribution were 978±422 mL/h/kg and 4.5±2.8 L/kg, respectively. | ||
Cladribine plasma concentration after intravenous administration declines multi-exponentially with an average half-life of 6.7 +/- 2.5 hours. In general, the apparent volume of distribution of cladribine is approximately 9 L/kg, indicating an extensive distribution in body tissues. | Cladribine plasma concentration after intravenous administration declines multi-exponentially with an average half-life of 6.7 +/- 2.5 hours. In general, the apparent volume of distribution of cladribine is approximately 9 L/kg, indicating an extensive distribution in body tissues. | ||
Cladribine penetrates into cerebrospinal fluid. One report indicates that concentrations are approximately 25% of those in plasma. | Cladribine penetrates into [[cerebrospinal fluid]]. One report indicates that concentrations are approximately 25% of those in plasma. | ||
Cladribine is bound approximately 20% to plasma proteins. | Cladribine is bound approximately 20% to plasma proteins. | ||
Except for some understanding of the mechanism of cellular toxicity, no other information is available on the metabolism of Cladribine in humans. An average of 18% of the administered dose has been reported to be excreted in urine of patients with solid tumors during a 5-day continuous intravenous infusion of 3.5-8.1 mg/m2/day of Cladribine. The effect of renal and hepatic impairment on the elimination of cladribine has not been investigated in humans. | Except for some understanding of the mechanism of cellular toxicity, no other information is available on the metabolism of Cladribine in humans. An average of 18% of the administered dose has been reported to be excreted in urine of patients with solid tumors during a 5-day continuous intravenous infusion of 3.5-8.1 mg/m2/day of Cladribine. The effect of renal and hepatic impairment on the elimination of cladribine has not been investigated in humans. | ||
|nonClinToxic=Carcinogenesis | |nonClinToxic=====Carcinogenesis==== | ||
No animal carcinogenicity studies have been conducted with cladribine. However, its carcinogenic potential cannot be excluded based on demonstrated genotoxicity of cladribine. | No animal carcinogenicity studies have been conducted with cladribine. However, its carcinogenic potential cannot be excluded based on demonstrated genotoxicity of cladribine. | ||
Mutagenesis | ====Mutagenesis==== | ||
As expected for compounds in this class, the actions of cladribine yield DNA damage. In mammalian cells in culture, cladribine caused the accumulation of DNA strand breaks. Cladribine was also incorporated into DNA of human lymphoblastic leukemia cells. Cladribine was not mutagenic in vitro (Ames and Chinese hamster ovary cell gene mutation tests) and did not induce unscheduled DNA synthesis in primary rat hepatocyte cultures. However, cladribine was clastogenic both in vitro (chromosome aberrations in Chinese hamster ovary cells) and in vivo (mouse bone marrow micronucleus test). | As expected for compounds in this class, the actions of cladribine yield [[DNA]] damage. In mammalian cells in culture, cladribine caused the accumulation of [[DNA]] strand breaks. Cladribine was also incorporated into [[DNA]] of human lymphoblastic leukemia cells. Cladribine was not mutagenic [[in vitro]] (Ames and Chinese hamster ovary cell gene mutation tests) and did not induce unscheduled [[DNA synthesis]] in primary rat hepatocyte cultures. However, cladribine was clastogenic both [[in vitro]] (chromosome aberrations in Chinese hamster ovary cells) and in vivo (mouse bone marrow micronucleus test). | ||
Impairment of Fertility | ====Impairment of Fertility==== | ||
When administered intravenously to Cynomolgus monkeys, cladribine has been shown to cause suppression of rapidly generating cells, including testicular cells. The effect on human fertility is unknown. | When administered intravenously to Cynomolgus monkeys, cladribine has been shown to cause suppression of rapidly generating cells, including testicular cells. The effect on human fertility is unknown. | ||
|clinicalStudies=Two single-center open label studies of Cladribine have been conducted in patients with Hairy Cell Leukemia with evidence of active disease requiring therapy. In the study conducted at the Scripps Clinic and Research Foundation (Study A), 89 patients were treated with a single course of Cladribine Injection given by continuous intravenous infusion for 7 days at a dose of 0.09 mg/kg/day. In the study conducted at the M.D. Anderson Cancer Center (Study B), 35 patients were treated with a 7-day continuous intravenous infusion of Cladribine Injection at a comparable dose of 3.6 mg/m2/day. A complete response (CR) required clearing of the peripheral blood and bone marrow of hairy cells and recovery of the hemoglobin to 12 g/dL, platelet count to 100 x 109/L, and absolute neutrophil count to 1500 x 106/L. A good partial response (GPR) required the same hematologic parameters as a complete response, and that fewer than 5% hairy cells remain in the bone marrow. A partial response (PR) required that hairy cells in the bone marrow be decreased by at least 50% from baseline and the same response for hematologic parameters as for complete response. A pathologic relapse was defined as an increase in bone marrow hairy cells to 25% of pretreatment levels. A clinical relapse was defined as the recurrence of cytopenias, specifically, decreases in hemoglobin ≥ 2 g/dL, ANC ≥ 25% or platelet counts ≥ 50,000. Patients who met the criteria for a complete response but subsequently were found to have evidence of bone marrow hairy cells (< 25% of pretreatment levels) were reclassified as partial responses and were not considered to be complete responses with relapse. | |clinicalStudies=Two single-center open label studies of Cladribine have been conducted in patients with [[Hairy Cell Leukemia]] with evidence of active disease requiring therapy. In the study conducted at the Scripps Clinic and Research Foundation (Study A), 89 patients were treated with a single course of Cladribine Injection given by continuous intravenous infusion for 7 days at a dose of 0.09 mg/kg/day. In the study conducted at the M.D. Anderson Cancer Center (Study B), 35 patients were treated with a 7-day continuous intravenous infusion of Cladribine Injection at a comparable dose of 3.6 mg/m2/day. A complete response (CR) required clearing of the peripheral blood and [[bone marrow]] of [[hairy cells]] and recovery of the hemoglobin to 12 g/dL, platelet count to 100 x 109/L, and absolute neutrophil count to 1500 x 106/L. A good partial response (GPR) required the same hematologic parameters as a complete response, and that fewer than 5% hairy cells remain in the [[bone marrow]]. A partial response (PR) required that hairy cells in the [[bone marrow]] be decreased by at least 50% from baseline and the same response for hematologic parameters as for complete response. A pathologic relapse was defined as an increase in [[bone marrow]] [[hairy cells]] to 25% of pretreatment levels. A clinical relapse was defined as the recurrence of [[cytopenias]], specifically, decreases in [[hemoglobin]] ≥ 2 g/dL, ANC ≥ 25% or [[platelet counts]] ≥ 50,000. Patients who met the criteria for a complete response but subsequently were found to have evidence of [[bone marrow]] [[hairy cells]] (< 25% of pretreatment levels) were reclassified as partial responses and were not considered to be complete responses with relapse. | ||
Among patients evaluable for efficacy (N=106), using the hematologic and bone marrow response criteria described above, the complete response rates in patients treated with Cladribine Injection were 65% and 68% for Study A and Study B, respectively, yielding a combined complete response rate of 66%. Overall response rates (i.e., Complete plus Good Partial plus Partial Responses) were 89% and 86% in Study A and Study B, respectively, for a combined overall response rate of 88% in evaluable patients treated with Cladribine Injection. | Among patients evaluable for efficacy (N=106), using the hematologic and [[bone marrow]] response criteria described above, the complete response rates in patients treated with Cladribine Injection were 65% and 68% for Study A and Study B, respectively, yielding a combined complete response rate of 66%. Overall response rates (i.e., Complete plus Good Partial plus Partial Responses) were 89% and 86% in Study A and Study B, respectively, for a combined overall response rate of 88% in evaluable patients treated with Cladribine Injection. | ||
Using an intent-to-treat analysis (N=123) and further requiring no evidence of splenomegaly as a criterion for CR (i.e., no palpable spleen on physical examination and≤ 13 cm on CT scan), the complete response rates for Study A and Study B were 54% and 53%, respectively, giving a combined CR rate of 54%. The overall response rates (CR + GPR + PR) were 90% and 85%, for Studies A and B, respectively, yielding a combined overall response rate of 89%. | Using an intent-to-treat analysis (N=123) and further requiring no evidence of splenomegaly as a criterion for CR (i.e., no palpable spleen on physical examination and≤ 13 cm on CT scan), the complete response rates for Study A and Study B were 54% and 53%, respectively, giving a combined CR rate of 54%. The overall response rates (CR + GPR + PR) were 90% and 85%, for Studies A and B, respectively, yielding a combined overall response rate of 89%. | ||
| Line 234: | Line 317: | ||
[[File:Cladribine Clinical studies.png|none|400px]] | [[File:Cladribine Clinical studies.png|none|400px]] | ||
In these studies, 60% of the patients had not received prior chemotherapy for Hairy Cell Leukemia or had undergone splenectomy as the only prior treatment and were receiving Cladribine as a first-line treatment. The remaining 40% of the patients received Cladribine as a second-line treatment, having been treated previously with other agents, including α-interferon and/or deoxycoformycin. The overall response rate for patients without prior chemotherapy was 92%, compared with 84% for previously treated patients. Cladribine is active in previously treated patients; however, retrospective analysis suggests that the overall response rate is decreased in patients previously treated with splenectomy or deoxycoformycin and in patients refractory to α-interferon. | In these studies, 60% of the patients had not received prior chemotherapy for [[Hairy Cell]] [[Leukemia]] or had undergone splenectomy as the only prior treatment and were receiving Cladribine as a first-line treatment. The remaining 40% of the patients received Cladribine as a second-line treatment, having been treated previously with other agents, including α-interferon and/or [[deoxycoformycin]]. The overall response rate for patients without prior [[chemotherapy]] was 92%, compared with 84% for previously treated patients. Cladribine is active in previously treated patients; however, retrospective analysis suggests that the overall response rate is decreased in patients previously treated with splenectomy or deoxycoformycin and in patients refractory to [[α-interferon]]. | ||
[[File:Cladribine clinical studies 2.png|none|400px]] | [[File:Cladribine clinical studies 2.png|none|400px]] | ||
After a reversible decline, normalization of peripheral blood counts (Hemoglobin >12.0 g/dL, Platelets >100 x 109/L, Absolute Neutrophil Count (ANC) >1500 x 106/L) was achieved by 92% of evaluable patients. The median time to normalization of peripheral counts was 9 weeks from the start of treatment (Range: 2 to 72). The median time to normalization of Platelet Count was 2 weeks, the median time to normalization of ANC was 5 weeks and the median time to normalization of Hemoglobin was 8 weeks. With normalization of Platelet Count and Hemoglobin, requirements for platelet and RBC transfusions were abolished after Months 1 and 2, respectively, in those patients with complete response. Platelet recovery may be delayed in a minority of patients with severe baseline thrombocytopenia. Corresponding to normalization of ANC, a trend toward a reduced incidence of infection was seen after the third month, when compared to the months immediately preceding Cladribine therapy. | After a reversible decline, normalization of peripheral blood counts ([[Hemoglobin]] >12.0 g/dL, [[Platelets]] >100 x 109/L, [[Absolute Neutrophil Count]] (ANC) >1500 x 106/L) was achieved by 92% of evaluable patients. The median time to normalization of peripheral counts was 9 weeks from the start of treatment (Range: 2 to 72). The median time to normalization of [[Platelet Count]] was 2 weeks, the median time to normalization of [[ANC]] was 5 weeks and the median time to normalization of Hemoglobin was 8 weeks. With normalization of [[Platelet Count]] and [[Hemoglobin]], requirements for [[platelet]] and [[RBC]] transfusions were abolished after Months 1 and 2, respectively, in those patients with complete response. [[Platelet]] recovery may be delayed in a minority of patients with severe baseline [[thrombocytopenia]]. Corresponding to normalization of [[ANC]], a trend toward a reduced incidence of infection was seen after the third month, when compared to the months immediately preceding Cladribine therapy. | ||
[[File:Cladribine clinical studies 3.png|none|400px]] | [[File:Cladribine clinical studies 3.png|none|400px]] | ||
For patients achieving a complete response, the median time to response (i.e., absence of hairy cells in bone marrow and peripheral blood together with normalization of peripheral blood parameters), measured from treatment start, was approximately 4 months. Since bone marrow aspiration and biopsy were frequently not performed at the time of peripheral blood normalization, the median time to complete response may actually be shorter than that which was recorded. At the time of data cut-off, the median duration of complete response was greater than 8 months and ranged to 25+ months. Among 93 responding patients, seven had shown evidence of disease progression at the time of the data cut-off. In four of these patients, disease was limited to the bone marrow without peripheral blood abnormalities (pathologic progression), while in three patients there were also peripheral blood abnormalities (clinical progression). Seven patients who did not respond to a first course of Cladribine received a second course of therapy. In the five patients who had adequate follow-up, additional courses did not appear to improve their overall response. | For patients achieving a complete response, the median time to response (i.e., absence of [[hairy cells]] in [[bone marrow]] and peripheral blood together with normalization of peripheral blood parameters), measured from treatment start, was approximately 4 months. Since [[bone marrow]] aspiration and biopsy were frequently not performed at the time of peripheral blood normalization, the median time to complete response may actually be shorter than that which was recorded. At the time of data cut-off, the median duration of complete response was greater than 8 months and ranged to 25+ months. Among 93 responding patients, seven had shown evidence of disease progression at the time of the data cut-off. In four of these patients, disease was limited to the bone marrow without peripheral blood abnormalities (pathologic progression), while in three patients there were also peripheral blood abnormalities (clinical progression). Seven patients who did not respond to a first course of Cladribine received a second course of therapy. In the five patients who had adequate follow-up, additional courses did not appear to improve their overall response. | ||
|howSupplied=Cladribine Injection is supplied as a sterile, preservative-free, isotonic solution containing 10 mg (1 mg/mL) of Cladribine as 10 mL filled into a single-use clear flint glass vial, individually boxed. NDC 67457-450-10. | |howSupplied=*Cladribine Injection is supplied as a sterile, preservative-free, isotonic solution containing 10 mg (1 mg/mL) of Cladribine as 10 mL filled into a single-use clear flint glass vial, individually boxed. NDC 67457-450-10. | ||
|storage=*Store in refrigerator at 2° to 8°C (36° to 46°F). Protect from light during storage. | |storage=*Store in refrigerator at 2° to 8°C (36° to 46°F). Protect from light during storage. | ||
|packLabel=[[File:Cladribine FDA label.png|none|450px]] | |packLabel=[[File:Cladribine FDA label.png|none|450px]] | ||
|alcohol=Alcohol-Cladribine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Cladribine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | |||
{{LabelImage | |||
|fileName=Cladribine package.png | |||
}} | }} | ||
Revision as of 06:15, 28 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Cladribine Injection should be administered under the supervision of a qualified physician experienced in the use of antineoplastic therapy. Suppression of bone marrow function should be anticipated. This is usually reversible and appears to be dose dependent. Serious neurological toxicity (including irreversible paraparesis and quadraparesis) has been reported in patients who received Cladribine Injection by continuous infusion at high doses (4 to 9 times the recommended dose for Hairy Cell Leukemia). Neurologic toxicity appears to demonstrate a dose relationship; however, severe neurological toxicity has been reported rarely following treatment with standard cladribine dosing regimens.
Acute nephrotoxicity has been observed with high doses of Cladribine (4 to 9 times the recommended dose for Hairy Cell Leukemia), especially when given concomitantly with other nephrotoxic agents/therapies.
|
Overview
Cladribine is an antineoplastic agent that is FDA approved for the treatment of active Hairy Cell Leukemia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Neutropenia, fever, infections.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Active Hairy Cell Leukemia
- Defined by clinically significant anemia, neutropenia, thrombocytopenia or disease-related symptoms
- Dosage: single course given by continuous infusion for 7 consecutive days at a dose of 0.09 mg/kg/day.
- If the patient does not respond to the initial course of Cladribine Injection for Hairy Cell Leukemia, it is unlikely that they will benefit from additional courses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cladribine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cladribine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cladribine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cladribine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cladribine in pediatric patients.
Contraindications
- Cladribine Injection is contraindicated in those patients who are hypersensitive to this drug or any of its components.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Cladribine Injection should be administered under the supervision of a qualified physician experienced in the use of antineoplastic therapy. Suppression of bone marrow function should be anticipated. This is usually reversible and appears to be dose dependent. Serious neurological toxicity (including irreversible paraparesis and quadraparesis) has been reported in patients who received Cladribine Injection by continuous infusion at high doses (4 to 9 times the recommended dose for Hairy Cell Leukemia). Neurologic toxicity appears to demonstrate a dose relationship; however, severe neurological toxicity has been reported rarely following treatment with standard cladribine dosing regimens.
Acute nephrotoxicity has been observed with high doses of Cladribine (4 to 9 times the recommended dose for Hairy Cell Leukemia), especially when given concomitantly with other nephrotoxic agents/therapies.
|
- Severe bone marrow suppression, including neutropenia, anemia and thrombocytopenia, has been commonly observed in patients treated with Cladribine, especially at high doses.
- At initiation of treatment, most patients in the clinical studies had hematologic impairment as a manifestation of active Hairy Cell Leukemia.
- Following treatment with Cladribine, further hematologic impairment occurred before recovery of peripheral blood counts began.
- During the first two weeks after treatment initiation, mean Platelet Count, ANC, and Hemoglobin concentration declined and subsequently increased with normalization of mean counts by Day 12, Week 5 and Week 8, respectively.
- The myelosuppressive effects of Cladribine were most notable during the first month following treatment.
- Forty-four percent (44%) of patients received transfusions with RBCs and 14% received transfusions with platelets during Month 1.
- Careful hematologic monitoring, especially during the first 4 to 8 weeks after treatment with Cladribine Injection, is recommended.
- Fever (T ≥ 100°F) was associated with the use of Cladribine in approximately two-thirds of patients (131/196) in the first month of therapy.
- Virtually all of these patients were treated empirically with parenteral antibiotics. Overall, 47% (93/196) of all patients had fever in the setting of neutropenia (ANC ≤ 1000), including 62 patients (32%) with severe neutropenia (i.e., ANC ≤ 500).
- In a Phase I investigational study using Cladribine in high doses (4 to 9 times the recommended dose for Hairy Cell Leukemia) as part of a bone marrow transplant conditioning regimen, which also included high dose cyclophosphamide and total body irradiation, acute nephrotoxicity and delayed onset neurotoxicity were observed.
- Thirty one (31) poor-risk patients with drug-resistant acute leukemia in relapse (29 cases) or non-Hodgkins Lymphoma (2 cases) received Cladribine for 7 to 14 days prior to bone marrow transplantation.
- During infusion, 8 patients experienced gastrointestinal symptoms.
- While the bone marrow was initially cleared of all hematopoietic elements, including tumor cells, leukemia eventually recurred in all treated patients. Within 7 to 13 days after starting treatment with Cladribine, 6 patients (19%) developed manifestations of renal dysfunction (e.g., acidosis, anuria, elevated serum creatinine, etc.) and 5 required dialysis.
- Several of these patients were also being treated with other medications having known nephrotoxic potential.
- Renal dysfunction was reversible in 2 of these patients. In the 4 patients whose renal function had not recovered at the time of death, autopsies were performed; in 2 of these, evidence of tubular damage was noted.
- Eleven (11) patients (35%) experienced delayed onset neurologic toxicity.
- In the majority, this was characterized by progressive irreversible motor weakness (paraparesis/quadriparesis) of the upper and/or lower extremities, first noted 35 to 84 days after starting high dose therapy with Cladribine.
- Non-invasive testing (electromyography and nerve conduction studies) was consistent with demyelinating disease. Severe neurologic toxicity has also been noted with high doses of another drug in this class.
Axonal peripheral polyneuropathy was observed in a dose escalation study at the highest dose levels (approximately 4 times the recommended dose for Hairy Cell Leukemia) in patients not receiving cyclophosphamide or total body irradiation.
- Severe neurological toxicity has been reported rarely following treatment with standard cladribine dosing regimens.
In patients with Hairy Cell Leukemia treated with the recommended treatment regimen (0.09 mg/kg/day for 7 consecutive days), there have been no reports of nephrologic toxicities.
- Of the 196 Hairy Cell Leukemia patients entered in the two trials, there were 8 deaths following treatment. Of these, 6 were of infectious etiology, including 3 pneumonias, and 2 occurred in the first month following Cladribine therapy. Of the 8 deaths, 6 occurred in previously treated patients who were refractory to α interferon.
- Benzyl alcohol is a constituent of the recommended diluent for the 7-day infusion solution. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants.
Adverse Reactions
Clinical Trials Experience
Safety data are based on 196 patients with Hairy Cell Leukemia: the original cohort of 124 patients plus an additional 72 patients enrolled at the same two centers after the original enrollment cutoff.
- In Month 1 of the Hairy Cell Leukemia clinical trials, severe neutropenia was noted in 70% of patients, fever in 69%, and infection was documented in 28%. Other adverse experiences reported frequently during the first 14 days after initiating treatment included: fatigue (45%), nausea (28%), rash (27%), headache (22%) and injection site reactions (19%). Most non-hematologic adverse experiences were mild to moderate in severity.
Myelosuppression was frequently observed during the first month after starting treatment. Neutropenia (ANC < 500 x 106/L) was noted in 70% of patients, compared with 26% in whom it was present initially. Severe anemia (Hemoglobin < 8.5 g/dL) developed in 37% of patients, compared with 10% initially and thrombocytopenia (Platelets < 20 x 109/L) developed in 12% of patients, compared to 4% in whom it was noted initially. During the first month, 54 of 196 patients (28%) exhibited documented evidence of infection. Serious infections (e.g., septicemia, pneumonia) were reported in 6% of all patients; the remainder were mild or moderate. Several deaths were attributable to infection and/or complications related to the underlying disease. During the second month, the overall rate of documented infection was 6%; these infections were mild to moderate and no severe systemic infections were seen. After the third month, the monthly incidence of infection was either less than or equal to that of the months immediately preceding Cladribine therapy. During the first month, 11% of patients experienced severe fever (i.e., ≥104°F). Documented infections were noted in fewer than one-third of febrile episodes. Of the 196 patients studied, 19 were noted to have a documented infection in the month prior to treatment. In the month following treatment, there were 54 episodes of documented infection: 23 (42%) were bacterial, 11 (20%) were viral and 11 (20%) were fungal. Seven (7) of 8 documented episodes of herpes zoster occurred during the month following treatment. Fourteen (14) of 16 episodes of documented fungal infections occurred in the first two months following treatment. Virtually all of these patients were treated empirically with antibiotics. Analysis of lymphocyte subsets indicates that treatment with cladribine is associated with prolonged depression of the CD4 counts. Prior to treatment, the mean CD4 count was 766/μL. The mean CD4 count nadir, which occurred 4 to 6 months following treatment, was 272/μL. Fifteen (15) months after treatment, mean CD4 counts remained below 500/μL. CD8 counts behaved similarly, though increasing counts were observed after 9 months. The clinical significance of the prolonged CD4 lymphopenia is unclear.
Another event of unknown clinical significance includes the observation of prolonged bone marrow hypocellularity. Bone marrow cellularity of < 35% was noted after 4 months in 42 of 124 patients (34%) treated in the two pivotal trials. This hypocellularity was noted as late as day 1010. It is not known whether the hypocellularity is the result of disease related marrow fibrosis or if it is the result of cladribine toxicity. There was no apparent clinical effect on the peripheral blood counts.
The vast majority of rashes were mild and occurred in patients who were receiving or had recently been treated with other medications (e.g., allopurinol or antibiotics) known to cause rash.
Most episodes of nausea were mild, not accompanied by vomiting, and did not require treatment with antiemetics. In patients requiring antiemetics, nausea was easily controlled, most frequently with chlorpromazine.
Adverse reactions reported during the first 2 weeks following treatment initiation (regardless of relationship to drug) by > 5% of patients included:
Body as a Whole
Gastrointestinal
- Nausea (28%)
- Decreased appetite (17%)
- Vomiting (13%)
- Diarrhea (10%)
- Constipation (9%)
- Abdominal pain (6%)
Hemic/Lymphatic
Nervous System
Cardiovascular System
- Edema (6%)
- Tachycardia (6%)
Respiratory System
- Abnormal breath sounds (11%)
- Cough (10%)
- Abnormal chest sounds (9%)
- Shortness of breath (7%)
Skin/Subcutaneous Tissue
Musculoskeletal System
- Myalgia (7%)
- Arthralgia (5%)
- Injection site reactions (9%) (i.e., redness, swelling, pain),
- Thrombosis (2%)
- Phlebitis (2%)
- Broken catheter (1%).
These appear to be related to the infusion procedure and/or indwelling catheter, rather than the medication or the vehicle.
From Day 15 to the last follow-up visit, the only events reported by > 5% of patients were: fatigue (11%), rash (10%), headache (7%), cough (7%), and malaise (5%). For a description of adverse reactions associated with use of high doses in non-Hairy Cell Leukemia patients.
Postmarketing Experience
The following additional adverse events have been reported since the drug became commercially available. These adverse events have been reported primarily in patients who received multiple courses of Cladribine Injection:
Hematologic
- Bone marrow suppression with prolonged pancytopenia, including some reports of aplastic anemia; hemolytic anemia, which was reported in patients with lymphoid malignancies, occurring within the first few weeks following treatment. Rare cases of myelodysplastic syndrome have been reported.
Hepatic
- Reversible, generally mild increases in bilirubin and transaminases.
Nervous System
- Neurological toxicity; however, severe neurotoxicity has been reported rarely following treatment with standard cladribine dosing regimens.
Respiratory System
- Pulmonary interstitial infiltrates; in most cases, an infectious etiology was identified.
Skin/Subcutaneous
- Urticaria
- Hypereosinophilia
- In isolated cases Stevens-Johnson and toxic epidermal necrolysis have been reported in patients who were receiving or had recently been treated with other medications (e.g., allopurinol or antibiotics) known to cause these syndromes.
Opportunistic infections have occurred in the acute phase of treatment due to the immunosuppression mediated by Cladribine Injection.
Drug Interactions
There are no known drug interactions with Cladribine Injection. Caution should be exercised if Cladribine Injection is administered before, after, or in conjunction with other drugs known to cause immunosuppression or myelosuppression.
Use in Specific Populations
Pregnancy
- Cladribine Injection should not be given during pregnancy.
- Cladribine is teratogenic in mice and rabbits and consequently has the potential to cause fetal harm when administered to a pregnant woman.
- A significant increase in fetal variations was observed in mice receiving 1.5 mg/kg/day (4.5 mg/m2) and increased resorptions, reduced litter size and increased fetal malformations were observed when mice received 3.0 mg/kg/day (9 mg/m2). Fetal death and malformations were observed in rabbits that received 3.0 mg/kg/day (33.0 mg/m2).
- No fetal effects were seen in mice at 0.5 mg/kg/day (1.5 mg/m2) or in rabbits at 1.0 mg/kg/day (11.0 mg/m2).
- Although there is no evidence of teratogenicity in humans due to Cladribine, other drugs which inhibit DNA synthesis (e.g., methotrexate and aminopterin) have been reported to be teratogenic in humans.
- Cladribine has been shown to be embryotoxic in mice when given at doses equivalent to the recommended dose.
- There are no adequate and well controlled studies in pregnant women. If Cladribine is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing age should be advised to avoid becoming pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cladribine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cladribine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from cladribine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug for the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
- In a Phase I study involving patients 1-21 years old with relapsed acute leukemia, Cladribine was given by continuous intravenous infusion in doses ranging from 3 to 10.7 mg/m2/day for 5 days (one-half to twice the dose recommended in Hairy Cell Leukemia).
- In this study, the dose-limiting toxicity was severe myelosuppression with profound neutropenia and thrombocytopenia.
- At the highest dose (10.7 mg/m2/day), 3 of 7 patients developed irreversible myelosuppression and fatal systemic bacterial or fungal infections. No unique toxicities were noted in this study
Geriatic Use
- Clinical studies of Cladribine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
- In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients.
Gender
There is no FDA guidance on the use of Cladribine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cladribine with respect to specific racial populations.
Renal Impairment
- There are inadequate data on dosing of patients with renal insufficiency.
- Development of acute renal insufficiency in some patients receiving high doses of Cladribine has been described.
Hepatic Impairment
- There are inadequate data on dosing of patients with hepatic insufficiency.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cladribine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cladribine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous Infusion
Monitoring
- Periodic assessment of peripheral blood counts, particularly during the first 4 to 8 weeks post-treatment, is recommended to detect the development of anemia, neutropenia and thrombocytopenia and for early detection of any potential sequelae (e.g., infection or bleeding). *As with other potent chemotherapeutic agents, monitoring of renal and hepatic function is also recommended, especially in patients with underlying kidney or liver dysfunction.
- Fever was a frequently observed side effect during the first month on study. Since the majority of fevers occurred in neutropenic patients, patients should be closely monitored during the first month of treatment and empiric antibiotics should be initiated as clinically indicated.
IV Compatibility
Preparation and Administration of Intravenous Solutions:
- Cladribine Injection must be diluted with the designated diluent prior to administration.
- Since the drug product does not contain any anti-microbial preservative or bacteriostatic agent, aseptic technique and proper environmental precautions must be observed in preparation of Cladribine Injection solutions.
To prepare a single daily dose:
- Add the calculated dose (0.09 mg/kg or 0.09 mL/kg) of Cladribine Injection to an infusion bag containing 500 mL of 0.9% Sodium Chloride Injection, USP.
- Infuse continuously over 24 hours.
- Repeat daily for a total of 7 consecutive days.
- The use of 5% dextrose as a diluent is not recommended because of increased degradation of cladribine.
- Admixtures of Cladribine Injection are chemically and physically stable for at least 24 hours at room temperature under normal room fluorescent light in PVC infusion containers.
- Since limited compatibility data are available, adherence to the recommended diluents and infusion systems is advised.
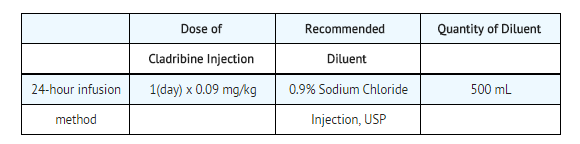
To prepare a 7-day infusion:
- The 7-day infusion solution should only be prepared with Bacteriostatic 0.9% Sodium Chloride Injection, USP (0.9% benzyl alcohol preserved).
- In order to minimize the risk of microbial contamination, both Cladribine Injection and the diluent should be passed through a sterile 0.22μ disposable hydrophilic syringe filter as each solution is being introduced into the infusion reservoir.
- First add the calculated dose of Cladribine Injection (7 days x 0.09 mg/kg or mL/kg) to the infusion reservoir through the sterile filter.
- Then add a calculated amount of Bacteriostatic 0.9% Sodium Chloride Injection, USP (0.9% benzyl alcohol preserved) also through the filter to bring the total volume of the solution to 100 mL.
- After completing solution preparation, clamp off the line, disconnect and discard the filter. *Aseptically aspirate air bubbles from the reservoir as necessary using the syringe and a dry second sterile filter or a sterile vent filter assembly.
- Reclamp the line and discard the syringe and filter assembly.
- Infuse continuously over 7 days.
- Solutions prepared with Bacteriostatic Sodium Chloride Injection for individuals weighing more than 85 kg may have reduced preservative effectiveness due to greater dilution of the benzyl alcohol preservative.
- Admixtures for the 7-day infusion have demonstrated acceptable chemical and physical stability for at least 7 days in the MEDICATION CASSETTE Reservoir.
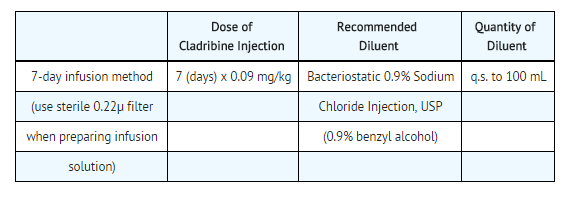
- Since limited compatibility data are available, adherence to the recommended diluents and infusion systems is advised.
- Solutions containing Cladribine Injection should not be mixed with other intravenous drugs or additives or infused simultaneously via a common intravenous line, since compatibility testing has not been performed.
- Preparations containing benzyl alcohol should not be used in neonates.
- Care must be taken to assure the sterility of prepared solutions.
- Once diluted, solutions of Cladribine Injection should be administered promptly or stored in the refrigerator (2° to 8° C) for no more than 8 hours prior to start of administration.
- Vials of Cladribine Injection are for single-use only. Any unused portion should be discarded in an appropriate manner
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- A precipitate may occur during the exposure of Cladribine Injection to low temperatures; it may be resolubilized by allowing the solution to warm naturally to room temperature and by shaking vigorously.
- DO NOT HEAT OR MICROWAVE.
Chemical Stability of Vials:
- When stored in refrigerated conditions between 2° to 8°C (36° to 46°F) protected from light, unopened vials of Cladribine Injection are stable until the expiration date indicated on the package.
- Freezing does not adversely affect the solution.
- If freezing occurs, thaw naturally to room temperature.
- DO NOT heat or microwave.
- Once thawed, the vial of Cladribine Injection is stable until expiry if refrigerated.
- DO NOT refreeze.
- Once diluted, solutions containing Cladribine Injection should be administered promptly or stored in the refrigerator (2° to 8°C) for no more than 8 hours prior to administration.
Handling and Disposal:
- The potential hazards associated with cytotoxic agents are well established and proper precautions should be taken when handling, preparing, and administering Cladribine Injection. *The use of disposable gloves and protective garments is recommended.
- If Cladribine Injection contacts the skin or mucous membranes, wash the involved surface immediately with copious amounts of water.
- Several guidelines on this subject have been published.(2-8) There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate. Refer to your Institution's guidelines and all applicable state/local regulations for disposal of cytotoxic waste.
Overdosage
High doses of Cladribine have been associated with:
- Irreversible neurologic toxicity (paraparesis/quadriparesis), acute nephrotoxicity, and severe bone marrow suppression resulting in neutropenia, anemia and thrombocytopenia.
- There is no known specific antidote to overdosage.
- Treatment of overdosage consists of discontinuation of Cladribine, careful observation and appropriate supportive measures.
- It is not known whether the drug can be removed from the circulation by dialysis or hemofiltration.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | Leustatin, Litak and Movectro |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693015 |
| Pregnancy category | |
| Routes of administration | Intravenous, subcutaneous, oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (i.v.) |
| Protein binding | 25% (range 5-50%)[1] |
| Metabolism | Mostly via intracellular kinases; 15-18% if excreted unchanged[1] |
| Elimination half-life | 5.4 hours (range 3-22 hours)[1] |
| Excretion | Urinary[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H12ClN5O3 |
| Molar mass | 285.687 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of Action
- The selective toxicity of 2-chloro-2΄-deoxy-β-D-adenosine towards certain normal and malignant lymphocyte and monocyte populations is based on the relative activities of deoxycytidine kinase and deoxynucleotidase.
- Cladribine passively crosses the cell membrane.
- In cells with a high ratio of deoxycytidine kinase to deoxynucleotidase, it is phosphorylated by deoxycytidine kinase to 2-chloro-2΄-deoxy- β -D-adenosine monophosphate (2-CdAMP). *Since 2-chloro-2΄-deoxy- β -D-adenosine is resistant to deamination by adenosine deaminase and there is little deoxynucleotide deaminase in lymphocytes and monocytes, 2-CdAMP accumulates intracellularly and is subsequently converted into the active triphosphate deoxynucleotide, 2-chloro-2΄-deoxy- β -D-adenosine triphosphate (2-CdATP).
- It is postulated that cells with high deoxycytidine kinase and low deoxynucleotidase activities will be selectively killed by 2-chloro-2΄-deoxy- β -D-adenosine as toxic deoxynucleotides accumulate intracellularly.
- Cells containing high concentrations of deoxynucleotides are unable to properly repair single-strand DNA breaks. The broken ends of DNA activate the enzyme poly (ADP-ribose) polymerase resulting in NAD and ATP depletion and disruption of cellular metabolism.
- There is evidence, also, that 2-CdATP is incorporated into the DNA of dividing cells, resulting in impairment of DNA synthesis.
- Thus, 2-chloro-2΄-deoxy- β -D-adenosine can be distinguished from other chemotherapeutic agents affecting purine metabolism in that it is cytotoxic to both actively dividing and quiescent lymphocytes and monocytes, inhibiting both DNA synthesis and repair.
Structure
Cladribine Injection (also commonly known as 2-chloro-2΄-deoxy- β -D-adenosine) is a synthetic antineoplastic agent for continuous intravenous infusion. It is a clear, colorless, sterile, preservative-free, isotonic solution. Cladribine Injection is available in single-use vials containing 10 mg (1 mg/mL) of cladribine, a chlorinated purine nucleoside analog. Each milliliter of Cladribine Injection contains 1 mg of the active ingredient and 9 mg (0.15 mEq) of sodium chloride as an inactive ingredient. The solution has a pH range of 5.5 to 8.0. Phosphoric acid and/or dibasic sodium phosphate may have been added to adjust the pH to 6.3±0.3.
The chemical name for cladribine is 2-chloro-6-amino-9-(2-deoxy-β-D-erythropento-furanosyl) purine and the structure is represented below:
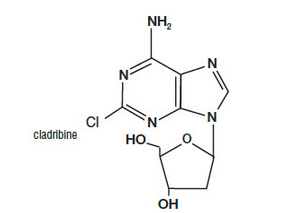
Pharmacodynamics
There is limited information regarding Cladribine Pharmacodynamics in the drug label.
Pharmacokinetics
In a clinical investigation, 17 patients with Hairy Cell Leukemia and normal renal function were treated for 7 days with the recommended treatment regimen of Cladribine Injection (0.09 mg/kg/day) by continuous intravenous infusion. The mean steady-state serum concentration was estimated to be 5.7 ng/mL with an estimated systemic clearance of 663.5 mL/h/kg when Cladribine was given by continuous infusion over 7 days. In Hairy Cell Leukemia patients, there does not appear to be a relationship between serum concentrations and ultimate clinical outcome.
In another study, 8 patients with hematologic malignancies received a two (2) hour infusion of Cladribine Injection (0.12 mg/kg). The mean end-of-infusion plasma Cladribine concentration was 48±19 ng/mL. For 5 of these patients, the disappearance of Cladribine could be described by either a biphasic or triphasic decline. For these patients with normal renal function, the mean terminal half-life was 5.4 hours. Mean values for clearance and steady-state volume of distribution were 978±422 mL/h/kg and 4.5±2.8 L/kg, respectively.
Cladribine plasma concentration after intravenous administration declines multi-exponentially with an average half-life of 6.7 +/- 2.5 hours. In general, the apparent volume of distribution of cladribine is approximately 9 L/kg, indicating an extensive distribution in body tissues.
Cladribine penetrates into cerebrospinal fluid. One report indicates that concentrations are approximately 25% of those in plasma.
Cladribine is bound approximately 20% to plasma proteins.
Except for some understanding of the mechanism of cellular toxicity, no other information is available on the metabolism of Cladribine in humans. An average of 18% of the administered dose has been reported to be excreted in urine of patients with solid tumors during a 5-day continuous intravenous infusion of 3.5-8.1 mg/m2/day of Cladribine. The effect of renal and hepatic impairment on the elimination of cladribine has not been investigated in humans.
Nonclinical Toxicology
Carcinogenesis
No animal carcinogenicity studies have been conducted with cladribine. However, its carcinogenic potential cannot be excluded based on demonstrated genotoxicity of cladribine.
Mutagenesis
As expected for compounds in this class, the actions of cladribine yield DNA damage. In mammalian cells in culture, cladribine caused the accumulation of DNA strand breaks. Cladribine was also incorporated into DNA of human lymphoblastic leukemia cells. Cladribine was not mutagenic in vitro (Ames and Chinese hamster ovary cell gene mutation tests) and did not induce unscheduled DNA synthesis in primary rat hepatocyte cultures. However, cladribine was clastogenic both in vitro (chromosome aberrations in Chinese hamster ovary cells) and in vivo (mouse bone marrow micronucleus test).
Impairment of Fertility
When administered intravenously to Cynomolgus monkeys, cladribine has been shown to cause suppression of rapidly generating cells, including testicular cells. The effect on human fertility is unknown.
Clinical Studies
Two single-center open label studies of Cladribine have been conducted in patients with Hairy Cell Leukemia with evidence of active disease requiring therapy. In the study conducted at the Scripps Clinic and Research Foundation (Study A), 89 patients were treated with a single course of Cladribine Injection given by continuous intravenous infusion for 7 days at a dose of 0.09 mg/kg/day. In the study conducted at the M.D. Anderson Cancer Center (Study B), 35 patients were treated with a 7-day continuous intravenous infusion of Cladribine Injection at a comparable dose of 3.6 mg/m2/day. A complete response (CR) required clearing of the peripheral blood and bone marrow of hairy cells and recovery of the hemoglobin to 12 g/dL, platelet count to 100 x 109/L, and absolute neutrophil count to 1500 x 106/L. A good partial response (GPR) required the same hematologic parameters as a complete response, and that fewer than 5% hairy cells remain in the bone marrow. A partial response (PR) required that hairy cells in the bone marrow be decreased by at least 50% from baseline and the same response for hematologic parameters as for complete response. A pathologic relapse was defined as an increase in bone marrow hairy cells to 25% of pretreatment levels. A clinical relapse was defined as the recurrence of cytopenias, specifically, decreases in hemoglobin ≥ 2 g/dL, ANC ≥ 25% or platelet counts ≥ 50,000. Patients who met the criteria for a complete response but subsequently were found to have evidence of bone marrow hairy cells (< 25% of pretreatment levels) were reclassified as partial responses and were not considered to be complete responses with relapse.
Among patients evaluable for efficacy (N=106), using the hematologic and bone marrow response criteria described above, the complete response rates in patients treated with Cladribine Injection were 65% and 68% for Study A and Study B, respectively, yielding a combined complete response rate of 66%. Overall response rates (i.e., Complete plus Good Partial plus Partial Responses) were 89% and 86% in Study A and Study B, respectively, for a combined overall response rate of 88% in evaluable patients treated with Cladribine Injection.
Using an intent-to-treat analysis (N=123) and further requiring no evidence of splenomegaly as a criterion for CR (i.e., no palpable spleen on physical examination and≤ 13 cm on CT scan), the complete response rates for Study A and Study B were 54% and 53%, respectively, giving a combined CR rate of 54%. The overall response rates (CR + GPR + PR) were 90% and 85%, for Studies A and B, respectively, yielding a combined overall response rate of 89%.
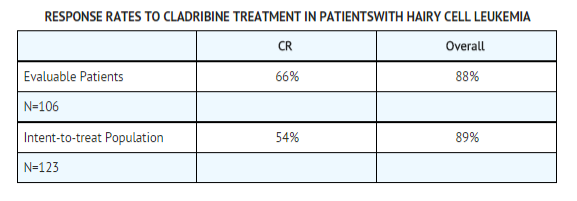
In these studies, 60% of the patients had not received prior chemotherapy for Hairy Cell Leukemia or had undergone splenectomy as the only prior treatment and were receiving Cladribine as a first-line treatment. The remaining 40% of the patients received Cladribine as a second-line treatment, having been treated previously with other agents, including α-interferon and/or deoxycoformycin. The overall response rate for patients without prior chemotherapy was 92%, compared with 84% for previously treated patients. Cladribine is active in previously treated patients; however, retrospective analysis suggests that the overall response rate is decreased in patients previously treated with splenectomy or deoxycoformycin and in patients refractory to α-interferon.
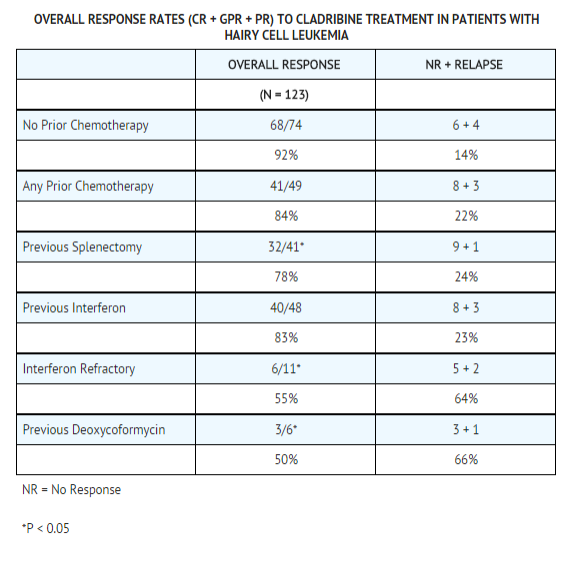
After a reversible decline, normalization of peripheral blood counts (Hemoglobin >12.0 g/dL, Platelets >100 x 109/L, Absolute Neutrophil Count (ANC) >1500 x 106/L) was achieved by 92% of evaluable patients. The median time to normalization of peripheral counts was 9 weeks from the start of treatment (Range: 2 to 72). The median time to normalization of Platelet Count was 2 weeks, the median time to normalization of ANC was 5 weeks and the median time to normalization of Hemoglobin was 8 weeks. With normalization of Platelet Count and Hemoglobin, requirements for platelet and RBC transfusions were abolished after Months 1 and 2, respectively, in those patients with complete response. Platelet recovery may be delayed in a minority of patients with severe baseline thrombocytopenia. Corresponding to normalization of ANC, a trend toward a reduced incidence of infection was seen after the third month, when compared to the months immediately preceding Cladribine therapy.
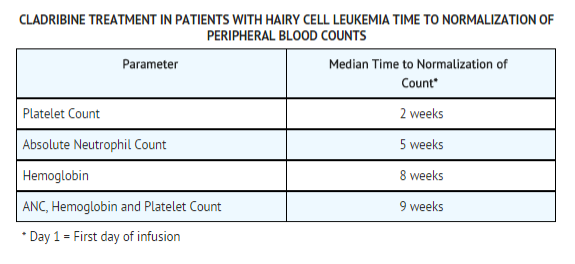
For patients achieving a complete response, the median time to response (i.e., absence of hairy cells in bone marrow and peripheral blood together with normalization of peripheral blood parameters), measured from treatment start, was approximately 4 months. Since bone marrow aspiration and biopsy were frequently not performed at the time of peripheral blood normalization, the median time to complete response may actually be shorter than that which was recorded. At the time of data cut-off, the median duration of complete response was greater than 8 months and ranged to 25+ months. Among 93 responding patients, seven had shown evidence of disease progression at the time of the data cut-off. In four of these patients, disease was limited to the bone marrow without peripheral blood abnormalities (pathologic progression), while in three patients there were also peripheral blood abnormalities (clinical progression). Seven patients who did not respond to a first course of Cladribine received a second course of therapy. In the five patients who had adequate follow-up, additional courses did not appear to improve their overall response.
How Supplied
- Cladribine Injection is supplied as a sterile, preservative-free, isotonic solution containing 10 mg (1 mg/mL) of Cladribine as 10 mL filled into a single-use clear flint glass vial, individually boxed. NDC 67457-450-10.
Storage
- Store in refrigerator at 2° to 8°C (36° to 46°F). Protect from light during storage.
Images
Drug Images
{{#ask: Page Name::Cladribine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cladribine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cladribine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cladribine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Cladribine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Cladribine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 "PRODUCT INFORMATION LITAK© 2 mg/mL solution for injection" (PDF). TGA eBusiness Services. St Leonards, Australia: Orphan Australia Pty. Ltd. 10 May 2010. Retrieved 27 November 2014.
{{#subobject:
|Label Page=Cladribine |Label Name=Cladribine package.png
}}