Chlorphenamine: Difference between revisions
No edit summary |
No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Chlorphenamine | |genericName=Chlorphenamine | ||
|aOrAn=an | |aOrAn=an | ||
|drugClass=anti- allergic agent | |drugClass=anti-allergic agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=allergic rhinitis | |indication=[[allergic rhinitis]] and [[common cold]] | ||
|adverseReactions=[[diarrhea]], [[nausea]], [[vomiting]], and [[somnolence]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 30: | Line 31: | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chlorphenamine in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Chlorphenamine in adult patients. | |||
|fdaLIADPed======Allergic rhinitis===== | |fdaLIADPed======Allergic rhinitis===== | ||
| Line 55: | Line 58: | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
| | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chlorphenamine in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Chlorphenamine in pediatric patients. | |||
< | |contraindications=Hypersensitivity to chlorpheniramine or dexchlorpheniramine | ||
|warnings=*Ask a doctor before use if you have a breathing problem such as emphysema or chronic bronchitis, glaucoma or trouble urinating due to an enlarged prostate gland | |warnings=*Ask a doctor before use if you have a breathing problem such as emphysema or chronic bronchitis, glaucoma or trouble urinating due to an enlarged prostate gland | ||
| Line 78: | Line 81: | ||
|drugInteractions= | |drugInteractions= | ||
* Almotriptan | |||
* Amitriptyline | |||
* Amoxapine | |||
* Belladonna | |||
* Belladonna Alkaloids | |||
* Bupropion | |||
* Desvenlafaxine | |||
* Dolasetron | |||
* Donepezil | |||
* Fentanyl | |||
* Fluoxetine | |||
* Fosphenytoin | |||
* Granisetron | |||
* Hydroxytryptophan | |||
* Levomilnacipran | |||
* Lorcaserin | |||
* Mepenzolate | |||
* Meperidine | |||
* Metoprolol | |||
* Mirtazapine | |||
* Morphine | |||
* Morphine Sulfate Liposome | |||
* Oxymorphone | |||
* Palonosetron | |||
* Phenytoin | |||
* Procarbazine | |||
* Tramadol | |||
* Trazodone | |||
* Umeclidinium | |||
* Vilazodone | |||
* Vortioxetine | |||
* Ziprasidone | |||
|useInPregnancyFDA=* '''Pregnancy Category''' | |useInPregnancyFDA=* '''Pregnancy Category''' | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 95: | Line 130: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|monitoring= | |monitoring=<!--IV Compatibility--> | ||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
| Line 102: | Line 136: | ||
|drugBox=[[File:Chlorpheniramine wiki.png|600px|thumbnail|left]] | |drugBox=[[File:Chlorpheniramine wiki.png|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
|mechAction=* | |mechAction=* | ||
| Line 121: | Line 153: | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies=<!--How Supplied--> | ||
<!--How Supplied--> | |||
|howSupplied=* | |howSupplied=* | ||
|packLabel=[[File:Chlorpheniramine pdp.jpg|600px|thumbnail|left]] | |packLabel=[[File:Chlorpheniramine pdp.jpg|600px|thumbnail|left]] | ||
Latest revision as of 15:45, 7 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chlorphenamine is an anti-allergic agent that is FDA approved for the treatment of allergic rhinitis and common cold. Common adverse reactions include diarrhea, nausea, vomiting, and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Allergic rhinitis
- Dosing Information
- The dosage of chlorpheniramine depends on the product. Use the following guidelines: 4-hour allergy tablet - 1 tablet (4 mg) every 4 to 6 hours up to a maximum of 6 tablets in 24 hours; 8-hour allergy tablet - 1 tablet (8 mg) every 8 to 12 hours up to 3 tablets in 24 hours; 12-hour tablet - 1 tablet (12 mg) every 12 hours up to 2 tablets in 24 hours.
- For the prevention of allergic rhinitis symptoms, administer chlorpheniramine 4 mg (syrup or plain, uncoated tablet) and increase 3 times daily every 3 days as tolerated until reaching 8 mg 3 times daily. Start before the allergy season (for ragweed - August 1st) and continue until inhalant allergen is gone (first frost in ragweed areas). Most patients develop tolerance to the sedative effect if dose is started low and increased in small increments (only when previous lower dose is tolerated).
Common cold
- Dosing Information
- For cough, 2 teaspoonfuls of Codeprex(TM) (10 mL; equivalent to codeine 40 mg and chlorpheniramine maleate 8 mg) ORALLY every 12 hours; maximum dose 4 teaspoonfuls in 24 hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorphenamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorphenamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Allergic rhinitis
- Dosing Information
- Reports of infant deaths have been associated with over-the-counter (OTC) cough and cold medications, and chlorpheniramine is commonly used as an antihistamine, alone or in combination with a cough suppressant, a decongestant, and/or an expectorant in the OTC cough and cold products. In January 2008, the US Food and Drug Administration issued a public health advisory recommending that cough and cold products not be used in children younger than 2 years due to the risk of serious and life-threatening effects.
Common cold
- Dosing Information
- Reports of infant deaths have been associated with over-the-counter (OTC) cough and cold medications, and chlorpheniramine is commonly used as an antihistamine, alone or in combination with a cough suppressant, a decongestant, and/or an expectorant in the OTC cough and cold products. In January 2008, the US Food and Drug Administration issued a public health advisory recommending that cough and cold products not be used in children younger than 2 years due to the risk of serious and life-threatening effects.
- Important Note:
- Chlorpheniramine may cause excitability in children. It is not recommended to give the 8-hour or 12-hour allergy tablets to children younger than 12 years and the 4-hour allergy tablets to children younger than 6 years. Codeprex(TM) is not recommended for children younger than 6 years.
- The dose for children ages 6 to 11 years, using the 4-hour allergy tablet, is half of the recommended adult dose. Therefore, 2 mg (break the 4 mg tablet in half) every 4 to 6 hours up to 12 mg in 24 hours. The 8-hour and 12-hour allergy tablets are not recommended.
- Oral:
- To treat children with allergic rhinitis, start with 2 mg and increase progressively to 12 mg/day.
- For cough, allergic rhinitis, and hay fever in children 12 years or older: 2 teaspoonfuls of Codeprex(TM) (10 mL; equivalent to codeine 40 mg and chlorpheniramine maleate 8 mg) ORALLY every 12 hours; maximum dose 4 teaspoonfuls in 24 hours.
- For cough, allergic rhinitis, and hay fever in children 6 to 12 years of age: 1 teaspoonful of Codeprex(TM) (5 mL; equivalent to codeine 20 mg and chlorpheniramine maleate 4 mg) ORALLY every 12 hours; maximum dose 2 teaspoonfuls in 24 hours.
- Maximum Dose:
- The maximum recommended dose for oral chlorpheniramine in children 6 to 11 years old is 12 mg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorphenamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorphenamine in pediatric patients.
Contraindications
Hypersensitivity to chlorpheniramine or dexchlorpheniramine
Warnings
- Ask a doctor before use if you have a breathing problem such as emphysema or chronic bronchitis, glaucoma or trouble urinating due to an enlarged prostate gland
- Ask a doctor or pharmacist before use if you
- are taking sedatives or tranquilizers
- When using this product
- marked drowsiness may occur avoid alcoholic beverages alcohol, sedatives, and tranquilizers may increase drowsiness be careful when drivinga motor vehicle or operating machinery
- excitability may occur especially in children
- If pregnant or breast-feeding
- ask a health professional before use
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Chlorphenamine Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Chlorphenamine Postmarketing Experience in the drug label.
Drug Interactions
- Almotriptan
- Amitriptyline
- Amoxapine
- Belladonna
- Belladonna Alkaloids
- Bupropion
- Desvenlafaxine
- Dolasetron
- Donepezil
- Fentanyl
- Fluoxetine
- Fosphenytoin
- Granisetron
- Hydroxytryptophan
- Levomilnacipran
- Lorcaserin
- Mepenzolate
- Meperidine
- Metoprolol
- Mirtazapine
- Morphine
- Morphine Sulfate Liposome
- Oxymorphone
- Palonosetron
- Phenytoin
- Procarbazine
- Tramadol
- Trazodone
- Umeclidinium
- Vilazodone
- Vortioxetine
- Ziprasidone
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chlorphenamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chlorphenamine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Chlorphenamine with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Chlorphenamine with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Chlorphenamine with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Chlorphenamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chlorphenamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chlorphenamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chlorphenamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chlorphenamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chlorphenamine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Chlorphenamine Administration in the drug label.
Monitoring
There is limited information regarding Chlorphenamine Monitoring in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Chlorphenamine in the drug label.
Overdosage
There is limited information regarding Chlorphenamine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
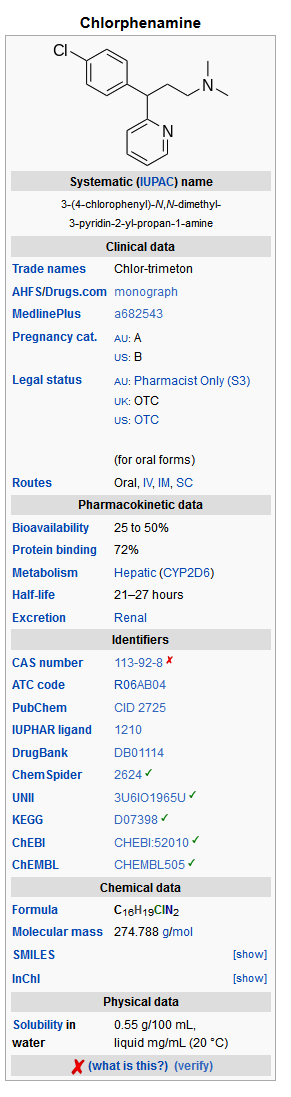
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Chlorphenamine in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Chlorphenamine in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Chlorphenamine in the drug label.
Clinical Studies
There is limited information regarding Chlorphenamine Clinical Studies in the drug label.
How Supplied
Storage
There is limited information regarding Chlorphenamine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Chlorphenamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
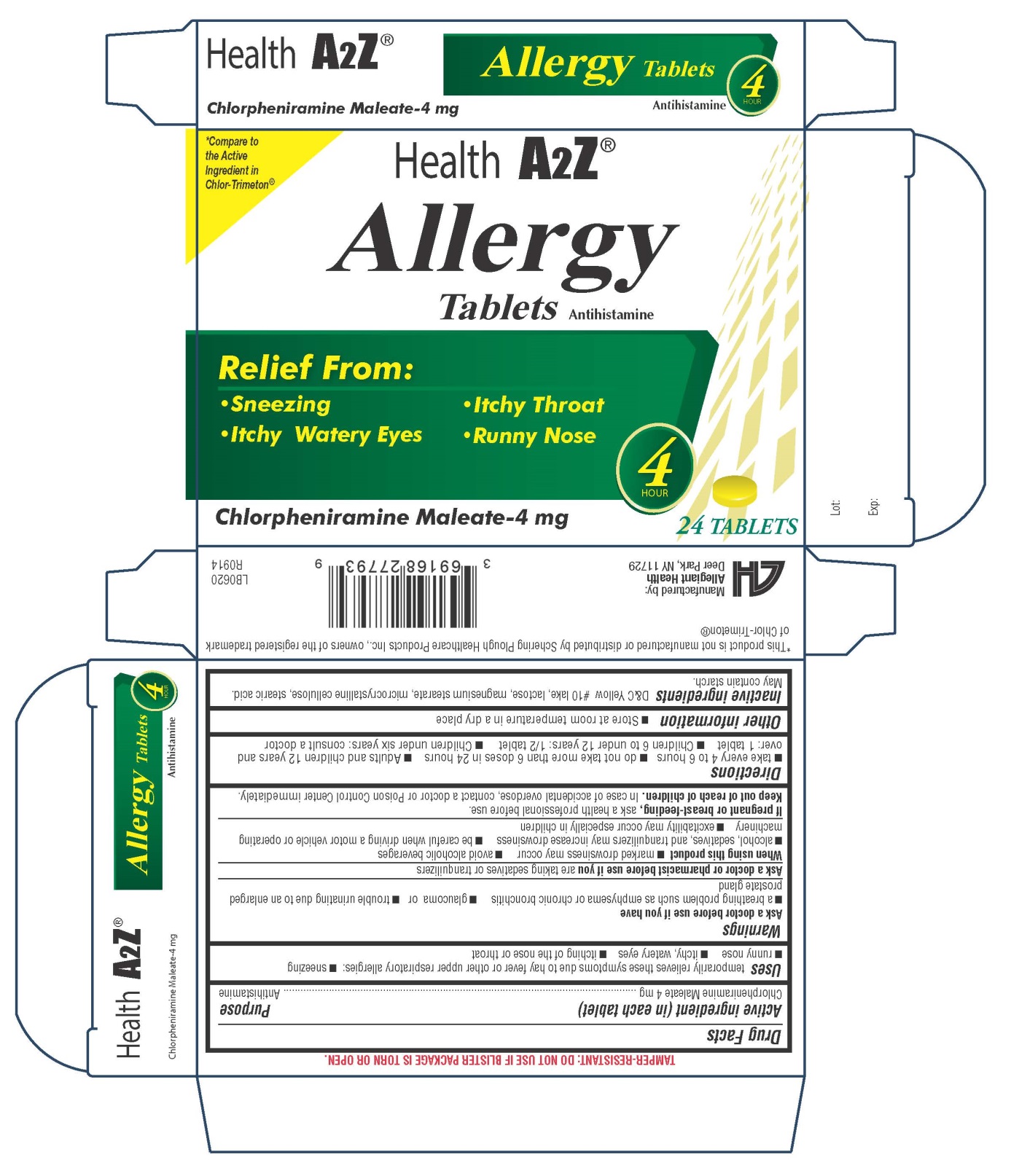
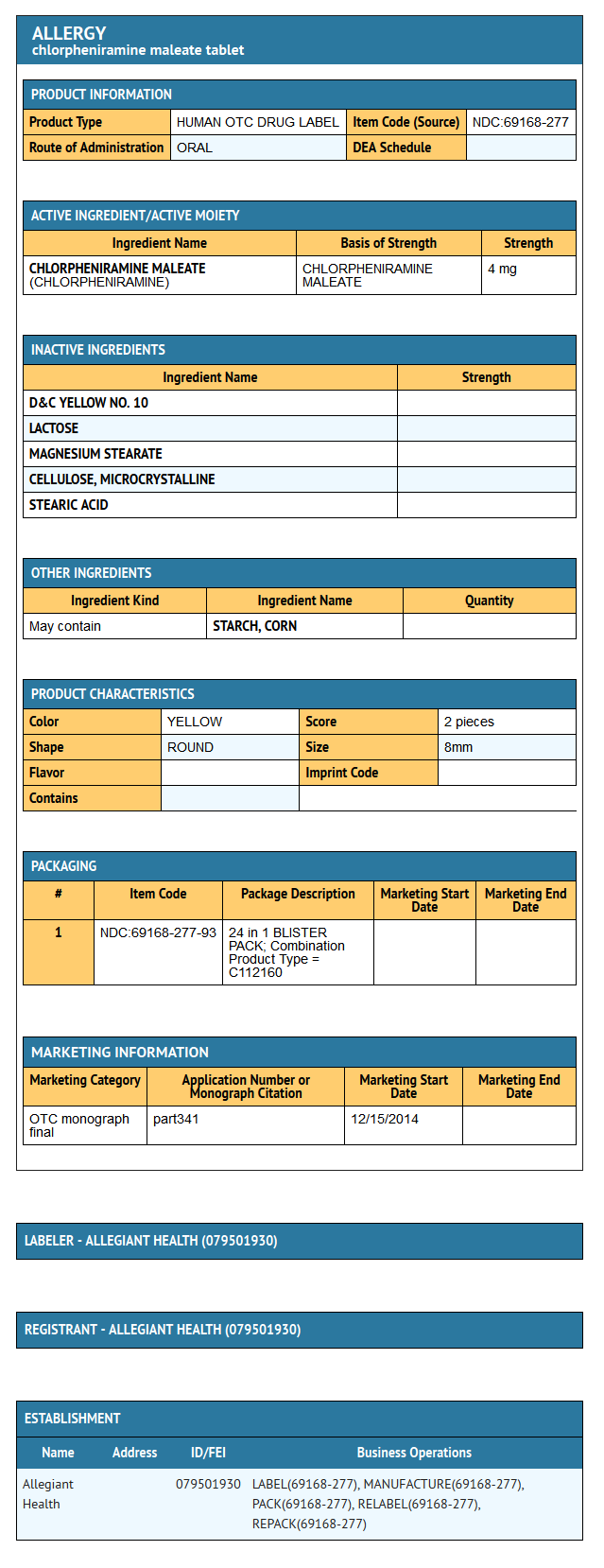
{{#ask: Label Page::Chlorphenamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Take every 4 to 6 hours do not take more than 6 doses in 24 hours Adults and children 12 years
and over: 1 tablet Children 6 to under 12 years: 1/2 tablet Children under 6 years: consult a doctor.
Precautions with Alcohol
- Alcohol-Chlorphenamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Aller-Chlor, C.P.M., Chlor-Phen, Chlor-Trimeton Allergy, Chlorphen, Diabetic Tussin Allergy Relief, Efidac 24 Chlorpheniramine, Teldrin HBP.
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Chlorphenamine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Chlorphenamine |Label Name=Chlorphenamine11.png
}}
{{#subobject:
|Label Page=Chlorphenamine |Label Name=Chlorphenamine11.png
}}