Chagas disease pathophysiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Chagas disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chagas disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chagas disease pathophysiology |
|
Risk calculators and risk factors for Chagas disease pathophysiology |
Overview
Infection cycle
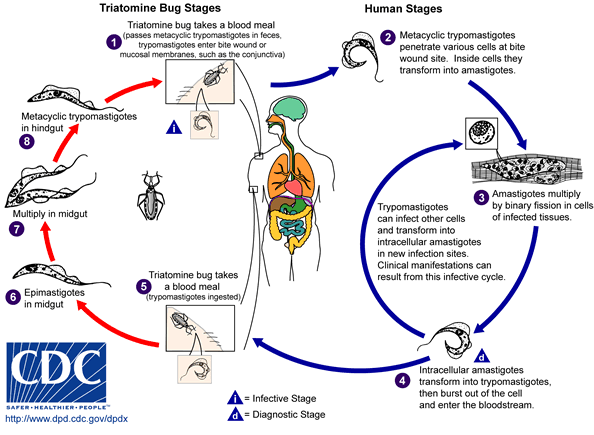
An infected triatomine insect vector feeds on blood and releases trypomastigotes in its feces near the site of the bite wound. The victim, by scratching the site of the bite, causes trypomastigotes to enter the host through the wound, or through intact mucosal membranes, such as the conjunctiva. Then, inside the host, the trypomastigotes invade cells, where they differentiate into intracellular amastigotes. The amastigotes multiply by binary fission and differentiate into trypomastigotes, then are released into the circulation as bloodstream trypomastigotes. These trypomastigotes infect cells from a variety of biological tissues and transform into intracellular amastigotes in new infection sites. Clinical manifestations and cell death at the target tissues can occur because of this infective cycle. For example, it has been shown by Austrian-Brazilian pathologist Dr. Fritz Köberle in the 1950s at the Medical School of the University of São Paulo at Ribeirão Preto, Brazil, that intracellular amastigotes destroy the intramural neurons of the autonomic nervous system in the intestine and heart, leading to megaintestine and heart aneurysms, respectively.
The bloodstream trypomastigotes do not replicate (unlike the African trypanosomes). Replication resumes only when the parasites enter another cell or are ingested by another vector. The “kissing” bug becomes infected by feeding on human or animal blood that contains circulating parasites. Moreover the bugs might be able to spread the infection to each other through their cannibalistic predatory behaviour. The ingested trypomastigotes transform into epimastigotes in the vector’s midgut. The parasites multiply and differentiate in the midgut and differentiate into infective metacyclic trypomastigotes in the hindgut.
Trypanosoma cruzi can also be transmitted through blood transfusions, organ transplantation, transplacentally, breast milk,[1] and in laboratory accidents. According to the World Health Organization, the infection rate in Latin American blood banks varies between 3% and 53%, a figure higher than of HIV infection and hepatitis B and C.[2]
Children can also acquire Chagas' Disease while still in the womb. Chagas' disease accounts for approximately 13% of stillborn deaths in parts of Brazil. It is recommended that pregnant women be tested for the disease.[3]
Alternative infection mechanism
Researchers suspected since 1991 that the transmission of the trypanosome by the oral route might be possible,[4] due to a number of micro-epidemics restricted to particular times and places (such as a farm or a family dwelling), particularly in non-endemic areas such as the Amazonia (17 such episodes recorded between 1968 and 1997). In 1991, farm workers in the state of Paraíba, Brazil, were apparently infected by contamination of food with opossum feces; and in 1997, in Macapá, state of Amapá, 17 members of two families were probably infected by drinking acai palm fruit juice contaminated with crushed triatomine vector insects.[5] In the beginning of 2005, a new outbreak with 27 cases was detected in Amapá. Despite many warnings in the press and by health authorities, this source of infection continues unabated. In August 2007 the Ministry of Health released the information that in the previous one year and half 15 clusters of Chagas infection in 116 people via ingestion of assai have been detected in the Amazon region [6]
In March 2005, a new startling outbreak was recorded in the state of Santa Catarina, Brazil, that seemed to confirm this alternative mechanism of transmission. Several people in Santa Catarina who had ingested sugar cane juice ("garapa", in Portuguese) by a roadside kiosk acquired Chagas' disease.[7] As of March 30, 2005, 49 cases had been confirmed in Santa Catarina, including 6 deaths. The hypothesized mechanism, so far, is that trypanosome-bearing insects were crushed into the raw preparation. The health authorities of Santa Catarina have estimated that ca. 60,000 people might have had contact with the contaminated food in Santa Catarina and urged everyone in this situation to submit to blood tests. They have prohibited the sale of sugar cane juice in the state until the situation is rectified.
The unusual severity of the disease outbreak has been blamed on a hypothetical higher parasite load achieved by the oral route of infection. Brazilian researchers at the Instituto Oswaldo Cruz, Rio de Janeiro, were able to infect mice via a gastrointestinal tube with trypanosome-infected oral preparations.
People also can become infected through:
- Consumption of uncooked food contaminated with feces from infected bugs.
- Congenital transmission (from a pregnant woman to her baby).
- Blood transfusion.
- Organ transplantation.
- Accidental laboratory exposure.
It is generally considered safe to breastfeed even if the mother has Chagas disease. However, if the mother has cracked nipples or blood in the breast milk, she should pump and discard the milk until the nipples heal and the bleeding resolves.
Chagas disease is not transmitted from person-to-person like a cold or the flu or through casual contact.
References
- ↑ Santos Ferreira C, Amato Neto V, Gakiya E, et al. "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321
- ↑ WHO. Chagas. Accessed 24 September 2006.
- ↑ Hudson L, Turner MJ. "Immunological Consequences of Infection and Vaccination in South American Trypanosomiasis [and Discussion]". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, Vol. 307, No. 1131, Towards the Immunological Control of Human Protozoal Diseases. (November 13, 1984), pp. 51–61. JSTOR. Accessed 2/22/07. PMID 6151688
- ↑ Shikanai-Yasuda MA, Marcondes CB, Guedes LA, et al. "Possible oral transmission of acute Chagas disease in Brazil." Rev Inst Med Trop São Paulo. 1991 Sep-Oct;33(5):351-7. PMID 1844961
- ↑ da Silva Valente S, de Costa Valente V, Neto H. "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon." Mem Inst Oswaldo Cruz 94 Suppl 1: 395-8. PMID 10677763
- ↑ Açaí faz 1 vítima de Chagas a cada 4 dias na Amazônia. Jornal Folha de São Paulo
- ↑ UK Health Protection Agency (HPA).Chagas’ disease (American trypanosomiasis) in southern Brazil. Accessed 24 September 2006.