Carboxypeptidase B2: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{redirect|TAFI||TAFI (disambiguation)}} | |||
{{selfref|On Wikipedia, "TAFI" refers to [[Wikipedia:Today's articles for improvement]].}} | |||
| | {{Infobox_gene}} | ||
| | |||
| | '''Carboxypeptidase B2''' ('''CPB2'''), also known as '''carboxypeptidase U''' ('''CPU'''), '''plasma carboxypeptidase B''' (pCPB) or '''thrombin-activatable fibrinolysis inhibitor''' ('''TAFI'''), is an [[enzyme]] that, in humans, is encoded by the [[gene]] ''CPB2''.<ref name="pmid1939207">{{cite journal |vauthors=Eaton DL, Malloy BE, Tsai SP, Henzel W, Drayna D |title=Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma |journal=J Biol Chem |volume=266 |issue=32 |pages=21833–8 |date=Dec 1991 |pmid=1939207 |pmc= |doi=}}</ref><ref name="pmid1427879">{{cite journal |vauthors=Tsai SP, Drayna D |title=The gene encoding human plasma carboxypeptidase B (CPB2) resides on chromosome 13 |journal=Genomics |volume=14 |issue=2 |pages=549–50 |date=Dec 1992 |pmid=1427879 |pmc= |doi=10.1016/S0888-7543(05)80268-X}}</ref> | ||
| | |||
==Function== | |||
}} | CPB2 is synthesized by the liver<ref name="Williams2010-8">Kaushansky K, Lichtman M, Beutler E, Kipps T, Prchal J, Seligsohn U. (2010; edition 8: pages 1833-1834 and 2040-2041) ''Williams Hematology''. McGraw-Hill. {{ISBN|978-0071621519}}</ref> and circulates in the plasma as a [[plasminogen]]-bound [[zymogen]]. When it is activated by proteolysis at residue Arg92 by the [[thrombin]]/[[thrombomodulin]] complex, CPB2 exhibits carboxypeptidase activity. Activated CPB2 reduces fibrinolysis by removing the [[fibrin]] [[C-terminus|C-terminal]] residues that are important for the binding and activation of [[plasminogen]].<ref name="pmid9869166">{{cite journal |vauthors=Zhao L, Morser J, Bajzar L, Nesheim M, Nagashima M | title = Identification and characterization of two thrombin-activatable fibrinolysis inhibitor isoforms | journal = Thromb. Haemost. | volume = 80 | issue = 6 | pages = 949–55 |date=December 1998 | pmid = 9869166 | doi = | url = }}</ref><ref name="pmid10350473">{{cite journal |vauthors=Boffa MB, Reid TS, Joo E, Nesheim ME, Koschinsky ML | title = Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B) | journal = Biochemistry | volume = 38 | issue = 20 | pages = 6547–58 |date=May 1999 | pmid = 10350473 | doi = 10.1021/bi990229v | url = }}</ref> | ||
{{ | [[Carboxypeptidase]]s are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by [[thrombin]] and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates [[fibrinolysis]].<ref name = "entrez">{{cite web | title = Entrez Gene: CPB2 carboxypeptidase B2 (plasma)| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1361| accessdate = }}</ref> | ||
{| | |||
|[[Image:Fibrinolysis.png|left|framed|Fibrinolysis (simplified). Blue arrows denote stimulation, and red arrows inhibition.]] | |||
|} | |||
==Isozymes== | |||
Polymorphisms have been described for this gene and its promoter region. Available sequence data analyses indicate splice variants that encode different isoforms.<ref name = "entrez"/> | |||
| | |||
==See also== | ==See also== | ||
*[[Carboxypeptidase B]] | *[[Carboxypeptidase B]] | ||
==References== | ==References== | ||
{{ | {{Reflist}} | ||
==Further reading== | ==Further reading== | ||
{{refbegin | 2}} | {{refbegin | 2}} | ||
{{PBB_Further_reading | {{PBB_Further_reading | ||
| citations = | | citations = | ||
*{{cite journal | | *{{cite journal |vauthors=Bouma BN, Mosnier LO |title=Thrombin activatable fibrinolysis inhibitor (TAFI) at the interface between coagulation and fibrinolysis. |journal=Pathophysiol. Haemost. Thromb. |volume=33 |issue= 5–6 |pages= 375–81 |year= 2005 |pmid= 15692247 |doi= 10.1159/000083832 }} | ||
*{{cite journal | | *{{cite journal |vauthors=Marinkovic DV, Marinkovic JN, Erdös EG, Robinson CJ |title=Purification of carboxypeptidase B from human pancreas |journal=Biochem. J. |volume=163 |issue= 2 |pages= 253–60 |year= 1977 |pmid= 17398 |doi= | pmc=1164691 }} | ||
*{{cite journal |vauthors=Pascual R, Burgos FJ, Salva M |title=Purification and properties of five different forms of human procarboxypeptidases |journal=Eur. J. Biochem. |volume=179 |issue= 3 |pages= 609–16 |year= 1989 |pmid= 2920728 |doi=10.1111/j.1432-1033.1989.tb14590.x |display-authors=etal}} | |||
*{{cite journal | | *{{cite journal |vauthors=Valnickova Z, Thogersen IB, Christensen S |title=Activated human plasma carboxypeptidase B is retained in the blood by binding to alpha2-macroglobulin and pregnancy zone protein |journal=J. Biol. Chem. |volume=271 |issue= 22 |pages= 12937–43 |year= 1996 |pmid= 8662763 |doi=10.1074/jbc.271.22.12937 |display-authors=etal}} | ||
*{{cite journal |vauthors=Bajzar L, Morser J, Nesheim M |title=TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex |journal=J. Biol. Chem. |volume=271 |issue= 28 |pages= 16603–8 |year= 1996 |pmid= 8663147 |doi=10.1074/jbc.271.28.16603 }} | |||
*{{cite journal | | *{{cite journal |vauthors=Vanhoof G, Wauters J, Schatteman K |title=The gene for human carboxypeptidase U (CPU)--a proposed novel regulator of plasminogen activation--maps to 13q14.11 |journal=Genomics |volume=38 |issue= 3 |pages= 454–5 |year= 1997 |pmid= 8975730 |doi= 10.1006/geno.1996.0656 |display-authors=etal}} | ||
*{{cite journal | | *{{cite journal |vauthors=Matsumoto A, Itoh K, Matsumoto R |title=A novel carboxypeptidase B that processes native beta-amyloid precursor protein is present in human hippocampus |journal=Eur. J. Neurosci. |volume=12 |issue= 1 |pages= 227–38 |year= 2000 |pmid= 10651877 |doi=10.1046/j.1460-9568.2000.00908.x }} | ||
*{{cite journal | | *{{cite journal |vauthors=Marx PF, Hackeng TM, Dawson PE |title=Inactivation of active thrombin-activable fibrinolysis inhibitor takes place by a process that involves conformational instability rather than proteolytic cleavage |journal=J. Biol. Chem. |volume=275 |issue= 17 |pages= 12410–5 |year= 2000 |pmid= 10777524 |doi=10.1074/jbc.275.17.12410 |display-authors=etal}} | ||
*{{cite journal |vauthors=Mosnier LO, Lisman T, van den Berg HM |title=The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration |journal=Thromb. Haemost. |volume=86 |issue= 4 |pages= 1035–9 |year= 2002 |pmid= 11686321 |doi= |display-authors=etal}} | |||
*{{cite journal | | *{{cite journal |vauthors=Mosnier LO, Meijers JC, Bouma BN |title=The role of protein S in the activation of thrombin activatable fibrinolysis inhibitor (TAFI) and regulation of fibrinolysis |journal=Thromb. Haemost. |volume=86 |issue= 4 |pages= 1040–6 |year= 2002 |pmid= 11686322 |doi= }} | ||
*{{cite journal |vauthors=Mosnier LO, Elisen MG, Bouma BN, Meijers JC |title=Protein C inhibitor regulates the thrombin-thrombomodulin complex in the up- and down regulation of TAFI activation |journal=Thromb. Haemost. |volume=86 |issue= 4 |pages= 1057–64 |year= 2002 |pmid= 11686324 |doi= }} | |||
*{{cite journal | | *{{cite journal |vauthors=Morange PE, Aillaud MF, Nicaud V |title=Ala147Thr and C+1542G polymorphisms in the TAFI gene are not associated with a higher risk of venous thrombosis in FV Leiden carriers |journal=Thromb. Haemost. |volume=86 |issue= 6 |pages= 1583–4 |year= 2002 |pmid= 11776333 |doi= |display-authors=etal}} | ||
*{{cite journal | | *{{cite journal |vauthors=Schneider M, Nagashima M, Knappe S |title=Amino acid residues in the P6-P'3 region of thrombin-activable fibrinolysis inhibitor (TAFI) do not determine the thrombomodulin dependence of TAFI activation |journal=J. Biol. Chem. |volume=277 |issue= 12 |pages= 9944–51 |year= 2002 |pmid= 11786552 |doi= 10.1074/jbc.M111685200 |display-authors=etal}} | ||
*{{cite journal | | *{{cite journal |vauthors=Koschinsky ML, Boffa MB, Nesheim ME |title=Association of a single nucleotide polymorphism in CPB2 encoding the thrombin-activable fibrinolysis inhibitor (TAF1) with blood pressure |journal=Clin. Genet. |volume=60 |issue= 5 |pages= 345–9 |year= 2002 |pmid= 11903334 |doi=10.1034/j.1399-0004.2001.600504.x |display-authors=etal}} | ||
*{{cite journal | | *{{cite journal |vauthors=Yano Y, Gabazza EC, Hori Y |title=Association between plasma thrombin-activatable fibrinolysis inhibitor levels and activated protein C in normotensive type 2 diabetic patients |journal=Diabetes Care |volume=25 |issue= 7 |pages= 1245–6 |year= 2002 |pmid= 12087030 |doi=10.2337/diacare.25.7.1245 |display-authors=etal}} | ||
*{{cite journal | | *{{cite journal |vauthors=Antovic JP, Blombäck M |title=Thrombin-activatable fibrinolysis inhibitor antigen and TAFI activity in patients with APC resistance caused by factor V Leiden mutation |journal=Thromb. Res. |volume=106 |issue= 1 |pages= 59–62 |year= 2003 |pmid= 12165290 |doi=10.1016/S0049-3848(02)00072-5 }} | ||
*{{cite journal | | |||
*{{cite journal | | |||
*{{cite journal | | |||
*{{cite journal | | |||
}} | }} | ||
{{refend}} | {{refend}} | ||
{{NLM content}} | {{NLM content}} | ||
{{Proteases}} | {{Proteases}} | ||
{{Coagulation}} | |||
{{PBB_Controls | |||
| update_page = yes | |||
| require_manual_inspection = no | |||
| update_protein_box = yes | |||
| update_summary = no | |||
| update_citations = no | |||
}} | |||
{{DEFAULTSORT:Thrombin-Activatable Fibrinolysis Inhibitor}} | |||
[[Category:Fibrinolytic system]] | |||
{{Hydrolase-stub}} | |||
Latest revision as of 20:46, 20 October 2017
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene CPB2.[1][2]
Function
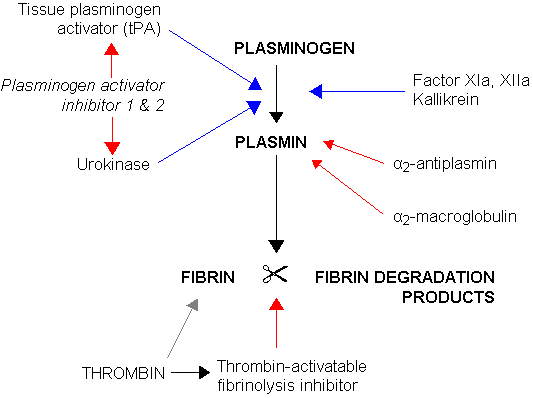
CPB2 is synthesized by the liver[3] and circulates in the plasma as a plasminogen-bound zymogen. When it is activated by proteolysis at residue Arg92 by the thrombin/thrombomodulin complex, CPB2 exhibits carboxypeptidase activity. Activated CPB2 reduces fibrinolysis by removing the fibrin C-terminal residues that are important for the binding and activation of plasminogen.[4][5]
Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by thrombin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis.[6]
 |
Isozymes
Polymorphisms have been described for this gene and its promoter region. Available sequence data analyses indicate splice variants that encode different isoforms.[6]
See also
References
- ↑ Eaton DL, Malloy BE, Tsai SP, Henzel W, Drayna D (Dec 1991). "Isolation, molecular cloning, and partial characterization of a novel carboxypeptidase B from human plasma". J Biol Chem. 266 (32): 21833–8. PMID 1939207.
- ↑ Tsai SP, Drayna D (Dec 1992). "The gene encoding human plasma carboxypeptidase B (CPB2) resides on chromosome 13". Genomics. 14 (2): 549–50. doi:10.1016/S0888-7543(05)80268-X. PMID 1427879.
- ↑ Kaushansky K, Lichtman M, Beutler E, Kipps T, Prchal J, Seligsohn U. (2010; edition 8: pages 1833-1834 and 2040-2041) Williams Hematology. McGraw-Hill. ISBN 978-0071621519
- ↑ Zhao L, Morser J, Bajzar L, Nesheim M, Nagashima M (December 1998). "Identification and characterization of two thrombin-activatable fibrinolysis inhibitor isoforms". Thromb. Haemost. 80 (6): 949–55. PMID 9869166.
- ↑ Boffa MB, Reid TS, Joo E, Nesheim ME, Koschinsky ML (May 1999). "Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B)". Biochemistry. 38 (20): 6547–58. doi:10.1021/bi990229v. PMID 10350473.
- ↑ 6.0 6.1 "Entrez Gene: CPB2 carboxypeptidase B2 (plasma)".
Further reading
- Bouma BN, Mosnier LO (2005). "Thrombin activatable fibrinolysis inhibitor (TAFI) at the interface between coagulation and fibrinolysis". Pathophysiol. Haemost. Thromb. 33 (5–6): 375–81. doi:10.1159/000083832. PMID 15692247.
- Marinkovic DV, Marinkovic JN, Erdös EG, Robinson CJ (1977). "Purification of carboxypeptidase B from human pancreas". Biochem. J. 163 (2): 253–60. PMC 1164691. PMID 17398.
- Pascual R, Burgos FJ, Salva M, et al. (1989). "Purification and properties of five different forms of human procarboxypeptidases". Eur. J. Biochem. 179 (3): 609–16. doi:10.1111/j.1432-1033.1989.tb14590.x. PMID 2920728.
- Valnickova Z, Thogersen IB, Christensen S, et al. (1996). "Activated human plasma carboxypeptidase B is retained in the blood by binding to alpha2-macroglobulin and pregnancy zone protein". J. Biol. Chem. 271 (22): 12937–43. doi:10.1074/jbc.271.22.12937. PMID 8662763.
- Bajzar L, Morser J, Nesheim M (1996). "TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex". J. Biol. Chem. 271 (28): 16603–8. doi:10.1074/jbc.271.28.16603. PMID 8663147.
- Vanhoof G, Wauters J, Schatteman K, et al. (1997). "The gene for human carboxypeptidase U (CPU)--a proposed novel regulator of plasminogen activation--maps to 13q14.11". Genomics. 38 (3): 454–5. doi:10.1006/geno.1996.0656. PMID 8975730.
- Matsumoto A, Itoh K, Matsumoto R (2000). "A novel carboxypeptidase B that processes native beta-amyloid precursor protein is present in human hippocampus". Eur. J. Neurosci. 12 (1): 227–38. doi:10.1046/j.1460-9568.2000.00908.x. PMID 10651877.
- Marx PF, Hackeng TM, Dawson PE, et al. (2000). "Inactivation of active thrombin-activable fibrinolysis inhibitor takes place by a process that involves conformational instability rather than proteolytic cleavage". J. Biol. Chem. 275 (17): 12410–5. doi:10.1074/jbc.275.17.12410. PMID 10777524.
- Mosnier LO, Lisman T, van den Berg HM, et al. (2002). "The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration". Thromb. Haemost. 86 (4): 1035–9. PMID 11686321.
- Mosnier LO, Meijers JC, Bouma BN (2002). "The role of protein S in the activation of thrombin activatable fibrinolysis inhibitor (TAFI) and regulation of fibrinolysis". Thromb. Haemost. 86 (4): 1040–6. PMID 11686322.
- Mosnier LO, Elisen MG, Bouma BN, Meijers JC (2002). "Protein C inhibitor regulates the thrombin-thrombomodulin complex in the up- and down regulation of TAFI activation". Thromb. Haemost. 86 (4): 1057–64. PMID 11686324.
- Morange PE, Aillaud MF, Nicaud V, et al. (2002). "Ala147Thr and C+1542G polymorphisms in the TAFI gene are not associated with a higher risk of venous thrombosis in FV Leiden carriers". Thromb. Haemost. 86 (6): 1583–4. PMID 11776333.
- Schneider M, Nagashima M, Knappe S, et al. (2002). "Amino acid residues in the P6-P'3 region of thrombin-activable fibrinolysis inhibitor (TAFI) do not determine the thrombomodulin dependence of TAFI activation". J. Biol. Chem. 277 (12): 9944–51. doi:10.1074/jbc.M111685200. PMID 11786552.
- Koschinsky ML, Boffa MB, Nesheim ME, et al. (2002). "Association of a single nucleotide polymorphism in CPB2 encoding the thrombin-activable fibrinolysis inhibitor (TAF1) with blood pressure". Clin. Genet. 60 (5): 345–9. doi:10.1034/j.1399-0004.2001.600504.x. PMID 11903334.
- Yano Y, Gabazza EC, Hori Y, et al. (2002). "Association between plasma thrombin-activatable fibrinolysis inhibitor levels and activated protein C in normotensive type 2 diabetic patients". Diabetes Care. 25 (7): 1245–6. doi:10.2337/diacare.25.7.1245. PMID 12087030.
- Antovic JP, Blombäck M (2003). "Thrombin-activatable fibrinolysis inhibitor antigen and TAFI activity in patients with APC resistance caused by factor V Leiden mutation". Thromb. Res. 106 (1): 59–62. doi:10.1016/S0049-3848(02)00072-5. PMID 12165290.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
| Stub icon | This hydrolase article is a stub. You can help Wikipedia by expanding it. |