Carboprost
For patient information regarding Carboprost tromethamine, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Carboprost is a Endocrine metabolic agent and prostaglandin that is FDA approved for the treatment of postpartum hemorrhage due to uterine atony, and aborting pregnancy related to second trimester. There is a Black Box Warning for this drug as shown here. Common adverse reactions include flushing, diarrhea, nausea, vomiting and leukocytosis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Carboprost tromethamine Sterile Solution is indicated for aborting pregnancy between the 13th and 20th weeks of gestation as calculated from the first day of the last normal menstrual period and in the following conditions related to second trimester abortion:
- Failure of expulsion of the fetus during the course of treatment by another method
- Premature rupture of membranes in intrauterine methods with loss of drug and insufficient or absent uterine activity
- Requirement of a repeat intrauterine instillation of drug for expulsion of the fetus
- Inadvertent or spontaneous rupture of membranes in the presence of a previable fetus and absence of adequate activity for expulsion.
- Carboprost tromethamine is indicated for the treatment of postpartum hemorrhage due to uterine atony which has not responded to conventional methods of management. Prior treatment should include the use of intravenously administered oxytocin, manipulative techniques such as uterine massage and, unless contraindicated, intramuscular ergot preparations. Studies have shown that in such cases, the use of Carboprost tromethamine has resulted in satisfactory control of hemorrhage, although it is unclear whether or not ongoing or delayed effects of previously administered ecbolic agents have contributed to the outcome. In a high proportion of cases, Carboprost tromethamine used in this manner has resulted in the cessation of life threatening bleeding and the avoidance of emergency surgical intervention.
Dosing information
Abortion and Indications 1–4
- An initial dose of 1 mL of Carboprost tromethamine Sterile Solution (containing the equivalent of 250 micrograms of carboprost) is to be administered deep in the muscle with a tuberculin syringe. Subsequent doses of 250 micrograms should be administered at 1½ to 3½ hour intervals depending on uterine response.
- An optional test dose of 100 micrograms (0.4 mL) may be administered initially. The dose may be increased to 500 micrograms (2 mL) if uterine contractility is judged to be inadequate after several doses of 250 micrograms (1 mL).
- he total dose administered of carboprost tromethamine should not exceed 12 milligrams and continuous administration of the drug for more than two days is not recommended.
For Refractory Postpartum Uterine Bleeding
- An initial dose of 250 micrograms of Carboprost tromethamine Sterile Solution (1 mL of Carboprost tromethamine) is to be given deep, intramuscularly. In clinical trials it was found that the majority of successful cases (73%) responded to single injections. In some selected cases, however, multiple dosing at intervals of 15 to 90 minutes was carried out with successful outcome. The need for additional injections and the interval at which these should be given can be determined only by the attending physicians as dictated by the course of clinical events. The total dose of Carboprost tromethamine should not exceed 2 milligrams (8 doses).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carboprost in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carboprost in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Carboprost in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carboprost in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carboprost in pediatric patients.
Contraindications
- Hypersensitivity (including anaphylaxis and angioedema) to Carboprost tromethamine Sterile Solution
- Acute pelvic inflammatory disease
- Patients with active cardiac, pulmonary, renal or hepatic disease
Warnings
- Carboprost tromethamine does not appear to directly affect the fetoplacental unit. Therefore, the possibility does exist that the previable fetus aborted by Carboprost tromethamine could exhibit transient life signs. Carboprost tromethamine is not indicated if the fetus in utero has reached the stage of viability. Carboprost tromethamine should not be considered a feticidal agent.
- Evidence from animal studies has suggested that certain other prostaglandins have some teratogenic potential. Although these studies do not indicate that Carboprost tromethamine is teratogenic, any pregnancy termination with Carboprost tromethamine that fails should be completed by some other means.
- This product contains benzyl alcohol. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants.
PRECAUTIONS
General
- Animal studies lasting several weeks at high doses have shown that prostaglandins of the E and F series can induce proliferation of bone. Such effects have also been noted in newborn infants who have received prostaglandin E1 during prolonged treatment. There is no evidence that short term administration of Carboprost tromethamine Sterile Solution can cause similar bone effects.
- In patients with a history of asthma, hypo- or hypertension, cardiovascular, renal, or hepatic disease, anemia, jaundice, diabetes, or epilepsy, Carboprost tromethamine should be used cautiously.
- As with any oxytocic agent, Carboprost tromethamine should be used with caution in patients with compromised (scarred) uteri.
Abortion
- As with spontaneous abortion, a process which is sometimes incomplete, abortion induced by Carboprost tromethamine may be expected to be incomplete in about 20% of cases.
- Although the incidence of cervical trauma is extremely small, the cervix should always be carefully examined immediately post-abortion.
- Use of Carboprost tromethamine is associated with transient pyrexia that may be due to its effect on hypothalamic thermoregulation. Temperature elevations exceeding 2° F (1.1° C) were observed in approximately one-eighth of the patients who received the recommended dosage regimen. In all cases, temperature returned to normal when therapy ended. Differentiation of post-abortion endometritis from drug-induced temperature elevations is difficult, but with increasing clinical experience, the distinctions become more obvious and are summarized below:
TABLE
Postpartum Hemorrhage
- Increased blood pressure. In the postpartum hemorrhage series, 5/115 (4%) of patients had an increase of blood pressure reported as a side effect. The degree of hypertension was moderate and it is not certain as to whether this was in fact due to a direct effect of Carboprost tromethamine or a return to a status of pregnancy associated hypertension manifest by the correction of hypovolemic shock. In any event the cases reported did not require specific therapy for the elevated blood pressure.
- Use in patients with chorioamnionitis. During the clinical trials with Carboprost tromethamine, chorioamnionitis was identified as a complication contributing to postpartum uterine atony and hemorrhage in 8/115 (7%) of cases, 3 of which failed to respond to Carboprost tromethamine. This complication during labor may have an inhibitory effect on the uterine response to Carboprost tromethamine similar to what has been reported for other oxytocic agents.1
- Duff, Sanders, and Gibbs; The course of labor in term patients with chorioamnionitis; Am. J. Obstet. Gynecol.; vol. 147, no. 4, October 15, 1983 pp 391–395.
Adverse Reactions
Clinical Trials Experience
- The adverse effects of Carboprost tromethamine Sterile Solution are generally transient and reversible when therapy ends. The most frequent adverse reactions observed are related to its contractile effect on smooth muscle.
- In patients studied, approximately two-thirds experienced vomiting and diarrhea, approximately one-third had nausea, one-eighth had a temperature increase greater than 2° F, and one-fourteenth experienced flushing.
- The pretreatment or concurrent administration of antiemetic and antidiarrheal drugs decreases considerably the very high incidence of gastrointestinal effects common with all prostaglandins used for abortion. Their use should be considered an integral part of the management of patients undergoing abortion with Carboprost tromethamine.
- Of those patients experiencing a temperature elevation, approximately one-sixteenth had a clinical diagnosis of endometritis. The remaining temperature elevations returned to normal within several hours after the last injection.
- Adverse effects observed during the use of Carboprost tromethamine for abortion and for hemorrhage, not all of which are clearly drug related, in decreasing order of frequency include:
TABLE
- The most common complications when Carboprost tromethamine was utilized for abortion requiring additional treatment after discharge from the hospital were endometritis, retained placental fragments, and excessive uterine bleeding, occurring in about one in every 50 patients.
Postmarketing Experience
- Hypersensitivity reactions (e.g. Anaphylactic reaction, Anaphylactic shock, Anaphylactoid reaction, Angioedema).
Drug Interactions
- Carboprost tromethamine may augment the activity of other oxytocic agents. Concomitant use with other oxytocic agents is not recommended.
Use in Specific Populations
Pregnancy
- Animal studies do not indicate that Carboprost tromethamine is teratogenic, however, it has been shown to be embryotoxic in rats and rabbits and any dose which produces increased uterine tone could put the embryo or fetus at risk.
Labor and Delivery
There is no FDA guidance on use of Carboprost during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Carboprost with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Carboprost with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Carboprost with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Carboprost with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carboprost with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carboprost in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carboprost in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carboprost in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carboprost in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular
Monitoring
There is limited information regarding Monitoring of Carboprost in the drug label.
- Description
Overdosage
There is limited information regarding Chronic Overdose of Carboprost in the drug label.
Pharmacology
| Template:Px | |
Carboprost
| |
| Systematic (IUPAC) name | |
| (5Z,9α,11α,13E,15S)-9,11,15-trihydroxy-15- methylprosta-5,13-dien-1-oic acid | |
| Identifiers | |
| CAS number | |
| ATC code | G02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 368.508 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
c |
| Legal status |
Template:Unicode Prescription only |
| Routes | Intramuscular |
Mechanism of Action
- Carboprost tromethamine administered intramuscularly stimulates in the gravid uterus myometrial contractions similar to labor contractions at the end of a full term pregnancy. Whether or not these contractions result from a direct effect of carboprost on the myometrium has not been determined. Nonetheless, they evacuate the products of conception from the uterus in most cases.
- Postpartum, the resultant myometrial contractions provide hemostasis at the site of placentation.
Structure
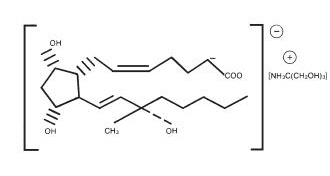
- Carboprost tromethamine Sterile Solution, an oxytocic, contains the tromethamine salt of the (15S)-15 methyl analogue of naturally occurring prostaglandin F2α in a solution suitable for intramuscular injection.
Carboprost tromethamine is the established name for the active ingredient in Carboprost tromethamine. Four other chemical names are:
1. (15S)-15-methyl prostaglandin F2α tromethamine salt
2. 7-(3α,5α-dihydroxy-2ß-[(3S)-3-hydroxy-3-methyl-trans-1-octenyl]-1α-cyclopentyl]-cis-5-heptenoic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
3. (15S)-9α,11α,15-trihydroxy-15-methylprosta-cis-5, trans-13-dienoic acid tromethamine salt
4. (15S)-15-methyl PGF2α-THAM
The structural formula is represented below:
Pharmacodynamics
- Carboprost tromethamine also stimulates the smooth muscle of the human gastrointestinal tract. This activity may produce the vomiting or diarrhea or both that is common when carboprost tromethamine is used to terminate pregnancy and for use postpartum. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost tromethamine used for the termination of pregnancy, and for use postpartum, some patients do experience transient temperature increases.
- In laboratory animals and in humans large doses of carboprost tromethamine can raise blood pressure, probably by contracting the vascular smooth muscle. With the doses of carboprost tromethamine used for terminating pregnancy, this effect has not been clinically significant. In laboratory animals and also in humans carboprost tromethamine can elevate body temperature. With the clinical doses of carboprost tromethamine used for the termination of pregnancy, some patients do experience temperature increases. In some patients, carboprost tromethamine may cause transient bronchoconstriction.
Pharmacokinetics
- Drug plasma concentrations were determined by radioimmunoassay in peripheral blood samples collected by different investigators from 10 patients undergoing abortion. The patients had been injected intramuscularly with 250 micrograms of carboprost at two hour intervals. Blood levels of drug peaked at an average of 2060 picograms/mL one-half hour after the first injection then declined to an average concentration of 770 picograms/mL two hours after the first injection just before the second injection. The average plasma concentration one-half hour after the second injection was slightly higher (2663 picograms/mL) than that after the first injection and decreased again to an average of 1047 picograms/mL by two hours after the second injection. Plasma samples were collected from 5 of these 10 patients following additional injections of the prostaglandin. The average peak concentrations of drug were slightly higher following each successive injection of the prostaglandin, but always decreased to levels less than the preceding peak values by two hours after each injection.
- Five women who had delivery spontaneously at term were treated immediately postpartum with a single injection of 250 micrograms of carboprost tromethamine. Peripheral blood samples were collected at several times during the four hours following treatment and carboprost tromethamine levels were determined by radioimmunoassay. The highest concentration of carboprost tromethamine was observed at 15 minutes in two patients (3009 and 2916 picograms/mL), at 30 minutes in two patients (3097 and 2792 picograms/mL), and at 60 minutes in one patient (2718 picograms/mL).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenic bioassay studies have not been conducted in animals with Carboprost tromethamine due to the limited indications for use and short duration of administration. No evidence of mutagenicity was observed in the Micronucleus Test or Ames Assay.
Clinical Studies
There is limited information regarding Clinical Studies of Carboprost in the drug label.
How Supplied
N/A
Images
Package and Label Display Panel
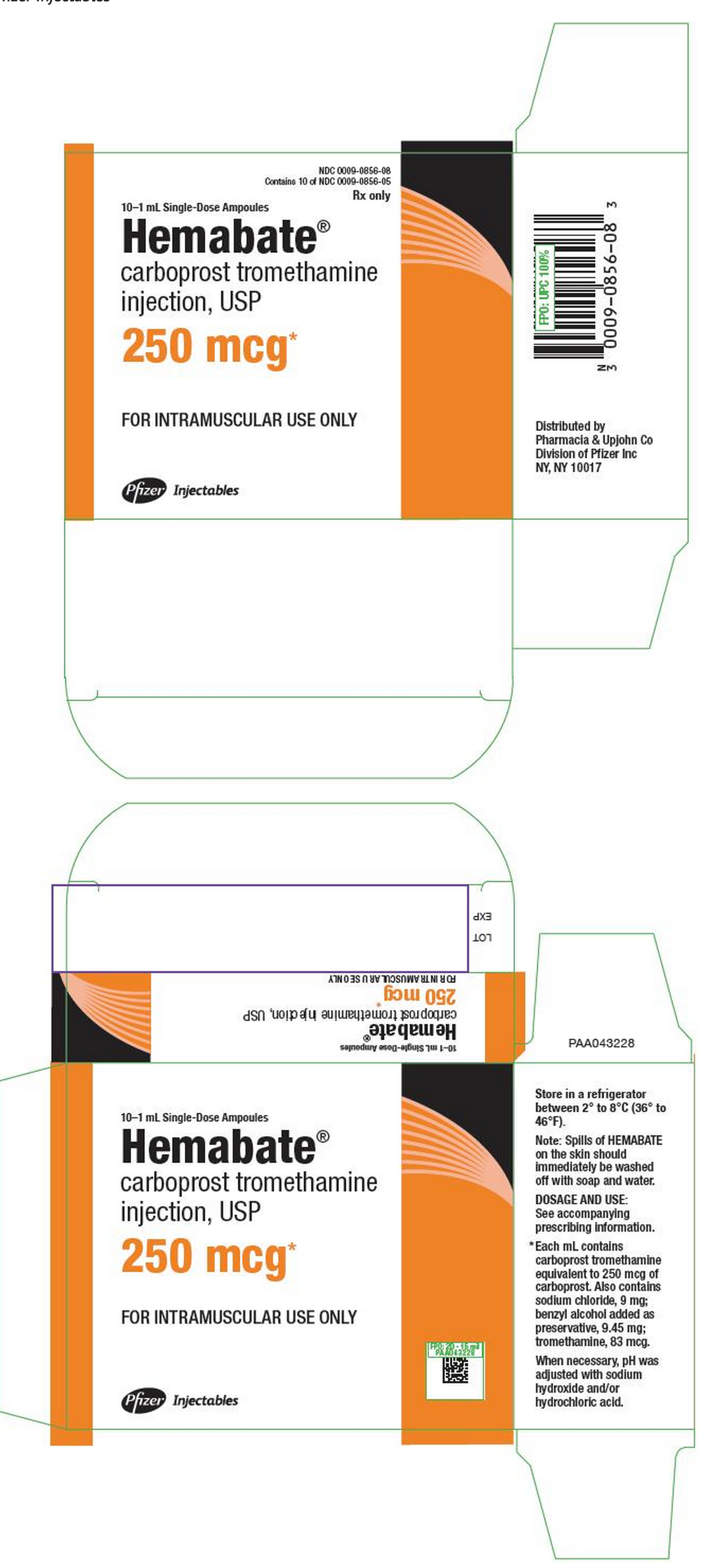
PRINCIPAL DISPLAY PANEL - 1 ML AMPOULE LABEL
NDC 0009-0856-08 Contains 10 of NDC 0009-0856-05
Rx only
10–1 mL Single-Dose Ampoules
Hemabate®
carboprost tromethamine injection, USP
250 mcg*
FOR INTRAMUSCULAR USE ONLY
Pfizer Injectables

Patient Counseling Information
There is limited information regarding Patient Counseling Information of Carboprost in the drug label.
Precautions with Alcohol
- Alcohol-Carboprost interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- HEMABATE®
Look-Alike Drug Names
N/A
Drug Shortage Status
References
The contents of this FDA label are provided by the National Library of Medicine.