Candida vulvovaginitis pathophysiology
|
Candidiasis Main page |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Dima Nimri, M.D. [2]
Overview
Pathophysiology
Pathogenesis
- All strains of C. albicans possess a yeast surface mannoprotein. This allows the various strains to adhere to both the exfoliated and buccal epithelial cells of the vagina.[1][2]
- Several virulence factors of Candida are implicated in vulvovaginitis. These include proteolytic enzymes, toxins and phospholipase. Proteolytic enzymes destroy the proteins that normally impair fungal invasion, allowing for Candida to colonize the vagina.[1][2][3]
- The understanding of the transition from asymptomatic vaginal colonization with Candida to symptomatic vulvovaginitis is not clear.[2][3]
Genetics
Genetic factors could be involved in the pathophysiology of Candida vulvovaginitis. Supporting evidence is that many cases were found to be more common in African-American women, run in families, as well as being associated with ABO-Lewis non-secretor phenotype, a rare blood group. In addition, women with Candida vulvovaginitis were found to have decreased concentrations of mannose binding lectin (MBL), hence, the variant (MBL) gene is thought to be a contributing factor in the development of Candida vulvovaginitis.[4][5][1]
Gross Pathology
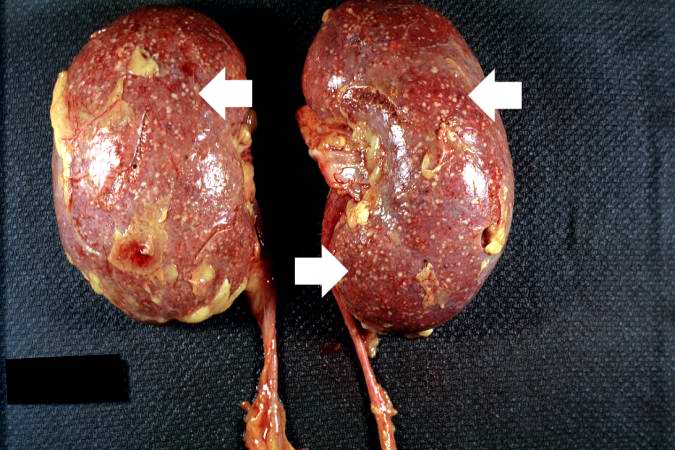
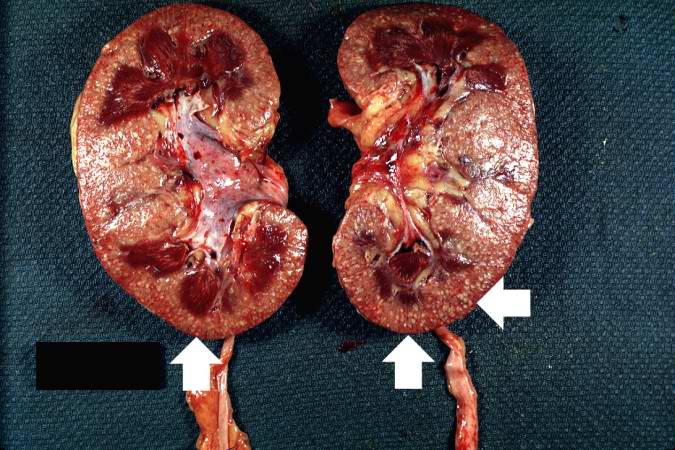
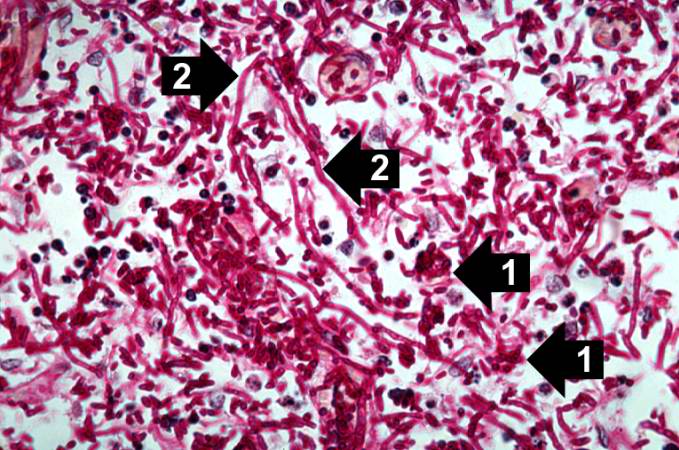
Microscopic Pathology
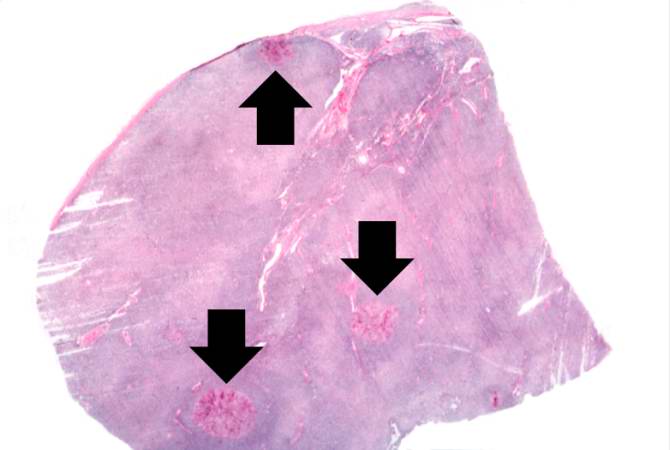
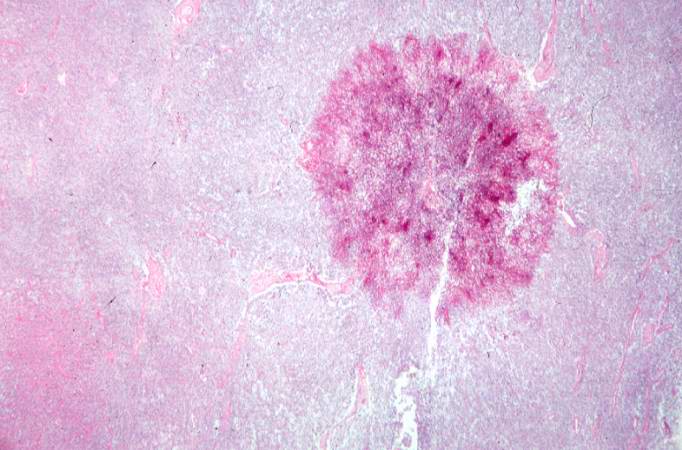


Associated Conditions
References
- ↑ 1.0 1.1 1.2 Sobel JD (2007). "Vulvovaginal candidosis". Lancet. 369 (9577): 1961–71. doi:10.1016/S0140-6736(07)60917-9. PMID 17560449.
- ↑ 2.0 2.1 2.2 Sobel JD (1985). "Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis". Am. J. Obstet. Gynecol. 152 (7 Pt 2): 924–35. PMID 3895958.
- ↑ 3.0 3.1 Sobel JD (1989). "Pathogenesis of Candida vulvovaginitis". Curr Top Med Mycol. 3: 86–108. PMID 2688924.
- ↑ Liu F, Liao Q, Liu Z (2006). "Mannose-binding lectin and vulvovaginal candidiasis". Int J Gynaecol Obstet. 92 (1): 43–7. doi:10.1016/j.ijgo.2005.08.024. PMID 16256117.
- ↑ Donders GG, Babula O, Bellen G, Linhares IM, Witkin SS (2008). "Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis". BJOG. 115 (10): 1225–31. doi:10.1111/j.1471-0528.2008.01830.x. PMID 18715406.