Cadherin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cadherins are a class of type-1 transmembrane proteins. They play important roles in cell adhesion, ensuring that cells within tissues are bound together. They are dependent on calcium (Ca2+) ions to function, hence their name.
The cadherin superfamily includes cadherins, protocadherins, desmogleins, and desmocollins, and more. In structure, they share cadherin repeats, which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecule, each designated with a one-letter prefix (generally noting the type of tissue with which it is associated). Cadherins within one class will bind only to themselves. For example, an N-cadherin will bind only to another N-cadherin molecule. Because of this specificity, groups of cells that express the same type of cadherin molecule tend to cluster together during development, whereas cells expressing different types of cadherin molecules tend to separate.
Types
Different members of the cadherin family are found in different locations. E-cadherins are found in epithelial tissue; N-cadherins are found in neurons; and P-cadherins are found in the placenta. T-cadherins have no cytoplasmic domains and must be tethered to the plasma membrane.
E-cadherin
| Cadherin 1, type 1, E-cadherin (epithelial) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
File:PBB Protein CDH1 image.jpg PDB rendering based on 1i7w. | |||||||||||
| Identifiers | |||||||||||
| Symbols | CDH1 ; Arc-1; CD324; CDHE; ECAD; LCAM; UVO | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 20917 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
| File:PBB GE CDH1 201131 s at tn.png | |||||||||||
| File:PBB GE CDH1 201130 s at tn.png | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
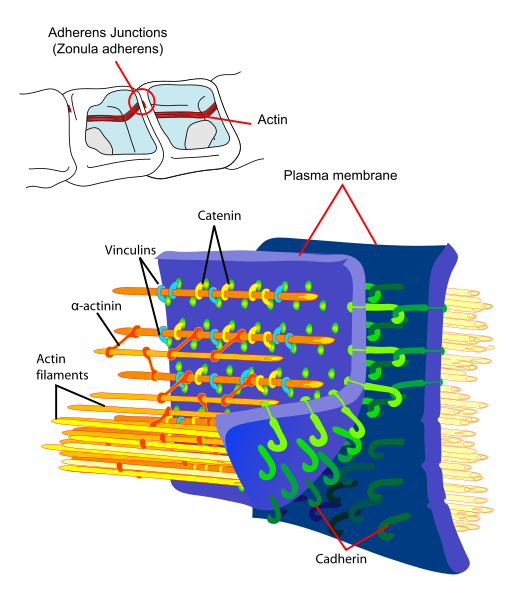
E-cadherin (epithelial) is probably the best understood cadherin. It consists of 5 cadherin repeats (EC1 ~ EC5) in the extracellular domain, one transmembrane domain, and an intracellular domain that binds p120-catenin and beta-catenin. The intracellular domain contains a highly-phosphorylated region vital to beta-catenin binding and therefore to E-cadherin function. Beta-catenin can also bind to alpha-catenin. Alpha-catenin participates in regulation of actin-containing cytoskeletal filaments. In epithelial cells, E-cadherin-containing cell-to-cell junctions are often adjacent to actin-containing filaments of the cytoskeleton.
E-cadherin is first expressed in the 2-cell stage of mammalian development, and becomes phosphorylated by the 8-cell stage, where it causes compaction. In adult tissues, E-cadherin is expressed in epithelial tissues, where it is constantly regenerated with a 5-hour half-life on the cell surface.
Loss of E-cadherin function or expression has been implicated in cancer progression and metastasis. E-cadherin downregulation decreases the strength of cellular adhesion within a tissue, resulting in an increase in cellular motility. This in turn may allow cancer cells to cross the basement membrane and invade surrounding tissues.
See also
- catenin
- protocadherin
- CDH2 - N-cadherin
- CDH4 - R-cadherin
- CDH6 - K-cadherin
- CDH11
- CDH13
- CDH15 - M-cadherin
- CDH16 - KSP-cadherin
- CDH17 - LI cadherin
- T-cadherin
- CDH5 - VE-cadherin
- CDH3 (gene) - P-cadherin
- DSC1, DSC2, DSC3 desmocollins
- Desmoglein 1, Desmoglein 2, Desmoglein 3
Further reading
- Berx G, Becker KF, Höfler H, van Roy F (1998). "Mutations of the human E-cadherin (CDH1) gene". Hum. Mutat. 12 (4): 226–37. doi:10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. PMID 9744472.
- Wijnhoven BP, Dinjens WN, Pignatelli M (2000). "E-cadherin-catenin cell-cell adhesion complex and human cancer". The British journal of surgery. 87 (8): 992–1005. doi:10.1046/j.1365-2168.2000.01513.x. PMID 10931041.
- Beavon IR (2000). "The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation". Eur. J. Cancer. 36 (13 Spec No): 1607–20. PMID 10959047.
- Wilson PD (2001). "Polycystin: new aspects of structure, function, and regulation". J. Am. Soc. Nephrol. 12 (4): 834–45. PMID 11274246.
- Chun YS, Lindor NM, Smyrk TC; et al. (2001). "Germline E-cadherin gene mutations: is prophylactic total gastrectomy indicated?". Cancer. 92 (1): 181–7. PMID 11443625.
- Hazan RB, Qiao R, Keren R; et al. (2004). "Cadherin switch in tumor progression". Ann. N. Y. Acad. Sci. 1014: 155–63. PMID 15153430.
- Bryant DM, Stow JL (2005). "The ins and outs of E-cadherin trafficking". Trends Cell Biol. 14 (8): 427–34. doi:10.1016/j.tcb.2004.07.007. PMID 15308209.
- Wang HD, Ren J, Zhang L (2004). "CDH1 germline mutation in hereditary gastric carcinoma". World J. Gastroenterol. 10 (21): 3088–93. PMID 15457549.
- Reynolds AB, Carnahan RH (2005). "Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer". Semin. Cell Dev. Biol. 15 (6): 657–63. doi:10.1016/j.semcdb.2004.09.003. PMID 15561585.
- Moran CJ, Joyce M, McAnena OJ (2005). "CDH1 associated gastric cancer: a report of a family and review of the literature". Eur J Surg Oncol. 31 (3): 259–64. doi:10.1016/j.ejso.2004.12.010. PMID 15780560.
- Georgolios A, Batistatou A, Manolopoulos L, Charalabopoulos K (2006). "Role and expression patterns of E-cadherin in head and neck squamous cell carcinoma (HNSCC)". J. Exp. Clin. Cancer Res. 25 (1): 5–14. PMID 16761612.
External links
- The cadherin family
- Online Textbook: "Molecular Biology of the Cell" by Bruce Alberts [2].
- The Cadherin Resource
- InterPro: IPR002126
ar:كادهيرين de:Cadherin it:Caderina nl:Cadherine Template:WH Template:WikiDoc Sources