Busulfan (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Busulfan is a potent cytotoxic drug that causes profound myelosuppression at the recommended dosage. It should be administered under the supervision of a qualified physician who is experienced in allogeneic hematopoietic stem cell transplantation, the use of cancer chemotherapeutic drugs and the management of patients with severe pancytopenia. Appropriate management of therapy and complications is only possible when adequate diagnostic and treatment facilities are readily available.
|
Overview
Busulfan (injection) is an alkylating agent that is FDA approved for the treatment of chronic myelogenous leukemia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, constipation, diarrhea, nausea, stomatitis, vomiting, asthenia, dizziness, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Busulfan is indicated for use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation for chronic myelogenous leukemia.
- Dosage: 0.8 mg/kg of ideal body weight or actual body weight, whichever is lower, administered every six hours for four days (a total of 16 doses).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Busulfan in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The effectiveness of busulfan in the treatment of CML has not been specifically studied in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Busulfan in pediatric patients.
Non–Guideline-Supported Use
- Acute myeloid leukemia undergoing bone marrow transplant
Contraindications
Busulfan is contraindicated in patients with a history of hypersensitivity to any of its components.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Busulfan is a potent cytotoxic drug that causes profound myelosuppression at the recommended dosage. It should be administered under the supervision of a qualified physician who is experienced in allogeneic hematopoietic stem cell transplantation, the use of cancer chemotherapeutic drugs and the management of patients with severe pancytopenia. Appropriate management of therapy and complications is only possible when adequate diagnostic and treatment facilities are readily available.
|
Busulfan should be administered under the supervision of a qualified physician experienced in hematopoietic stem cell transplantation. Appropriate management of complications arising from its administration is possible only when adequate diagnostic and treatment facilities are readily available.
The following warnings pertain to different physiologic effects of busulfan in the setting of allogeneic transplantation.
Hematologic
The most frequent serious consequence of treatment with busulfan at the recommended dose and schedule is profound myelosuppression, occurring in all patients. Severe granulocytopenia, thrombocytopenia, anemia, or any combination thereof may develop. Frequent complete blood counts, including white blood cell differentials, and quantitative platelet counts should be monitored during treatment and until recovery is achieved. Absolute neutrophil counts dropped below 0.5×109/L at a median of 4 days post-transplant in 100% of patients treated in the busulfan clinical trial. The absolute neutrophil count recovered at a median of 13 days following allogeneic transplantation when prophylactic G-CSF was used in the majority of patients. Thrombocytopenia (<25,000/mm3 or requiring platelet transfusion) occurred at a median of 5-6 days in 98% of patients. Anemia (hemoglobin <8.0 g/dL) occurred in 69% of patients. Antibiotic therapy and platelet and red blood cell support should be used when medically indicated.
Neurological
Seizures have been reported in patients receiving high-dose oral busulfan at doses producing plasma drug levels similar to those achieved following the recommended dosage of busulfan. Despite prophylactic therapy with phenytoin, one seizure (1/42 patients) was reported during an autologous transplantation clinical trial of busulfan. This episode occurred during the cyclophosphamide portion of the conditioning regimen, 36 hours after the last busulfan dose. Anti-convulsant prophylactic therapy should be initiated prior to busulfan treatment. Caution should be exercised when administering the recommended dose of busulfan to patients with a history of a seizure disorder or head trauma or who are receiving other potentially epileptogenic drugs.
Hepatic
Current literature suggests that high busulfan area under the plasma concentration verses time curve (AUC) values (>1,500 µM∙min) may be associated with an increased risk of developing hepatic veno-occlusive disease (HVOD). Patients who have received prior radiation therapy, greater than or equal to three cycles of chemotherapy, or a prior progenitor cell transplant may be at an increased risk of developing HVOD with the recommended busulfan dose and regimen. Based on clinical examination and laboratory findings, hepatic veno-occlusive disease was diagnosed in 8% (5/61) of patients treated with busulfan in the setting of allogeneic transplantation, was fatal in 2/5 cases (40%), and yielded an overall mortality from HVOD in the entire study population of 2/61 (3%). Three of the five patients diagnosed with HVOD were retrospectively found to meet the Jones criteria. The incidence of HVOD reported in the literature from the randomized, controlled trials (see CLINICAL STUDIES) was 7.7%-12%.
Cardiac
Cardiac tamponade has been reported in pediatric patients with thalassemia (8/400 or 2% in one series) who received high doses of oral busulfan and cyclophosphamide as the preparatory regimen for hematopoietic progenitor cell transplantation. Six of the eight children died and two were saved by rapid pericardiocentesis. Abdominal pain and vomiting preceded the tamponade in most patients. No patients treated in the busulfan (busulfan) Injection clinical trials experienced cardiac tamponade.
Pulmonary
Bronchopulmonary dysplasia with pulmonary fibrosis is a rare but serious complication following chronic busulfan therapy. The average onset of symptoms is 4 years after therapy (range 4 months to 10 years).
Adverse Reactions
Clinical Trials Experience
In the busulfan allogeneic stem cell transplantation clinical trial, all patients were treated with busulfan 0.8 mg/kg as a two‑hour infusion every six hours for 16 doses over four days, combined with cyclophosphamide 60 mg/kg ×2 days. Ninety-three percent (93%) of evaluable patients receiving this dose of busulfan maintained an AUC less than 1,500 µM∙min for dose 9, which has generally been considered the level that minimizes the risk of HVOD.

Hematologic
At the indicated dose and schedule, busulfan produced profound myelosuppression in 100% of patients. Following hematopoietic progenitor cell infusion, recovery of neutrophil counts to ≥500 cells/mm3 occurred at median day 13 when prophylactic G-CSF was administered to the majority of participants on the study. The median number of platelet transfusions per patient on study was 6, and the median number of red blood cell transfusions on study was 4. Prolonged prothrombin time was reported in one patient (2%).
Gastrointestinal
Gastrointestinal toxicities were frequent and generally considered to be related to the drug. Few were categorized as serious. Mild or moderate nausea occurred in 92% of patients in the allogeneic clinical trial, and mild or moderate vomiting occurred in 95% through BMT Day +28; nausea was severe in 7%. The incidence of vomiting during busulfan administration (BMT Day –7 to –4) was 43% in the allogeneic clinical trial. Grade 3-4 stomatitis developed in 26% of the participants, and Grade 3 esophagitis developed in 2%. Grade 3-4 diarrhea was reported in 5% of the allogeneic study participants, while mild or moderate diarrhea occurred in 75%. Mild or moderate constipation occurred in 38% of patients; ileus developed in 8% and was severe in 2%. Forty-four percent (44%) of patients reported mild or moderate dyspepsia. Two percent (2%) of patients experienced mild hematemesis. Pancreatitis developed in 2% of patients. Mild or moderate rectal discomfort occurred in 24% of patients. Severe anorexia occurred in 21% of patients and was mild/moderate in 64%.
Hepatic
Hyperbilirubinemia occurred in 49% of patients in the allogeneic BMT trial. Grade 3/4 hyperbilirubinemia occurred in 30% of patients within 28 days of transplantation and was considered life-threatening in 5% of these patients. Hyperbilirubinemia was associated with graft-versus-host disease in six patients and with hepatic veno-occlusive disease in 5 patients. Grade 3/4 SGPT elevations occurred in 7% of patients. Alkaline phosphatase increases were mild or moderate in 15% of patients. Mild or moderate jaundice developed in 12% of patients, and mild or moderate hepatomegaly developed in 6%.
Hepatic veno-occlusive disease
Hepatic veno-occlusive disease (HVOD) is a recognized potential complication of conditioning therapy prior to transplant. Based on clinical examination and laboratory findings, hepatic veno-occlusive disease was diagnosed in 8% (5/61) of patients treated with busulfan in the setting of allogeneic transplantation, was fatal in 2/5 cases (40%), and yielded an overall mortality from HVOD in the entire study population of 2/61 (3%). Three of the five patients diagnosed with HVOD were retrospectively found to meet the Jones criteria.
Graft-versus-host disease
Graft-versus-host disease developed in 18% of patients (11/61) receiving allogeneic transplants; it was severe in 3%, and mild or moderate in 15%. There were 3 deaths (5%) attributed to GVHD.
Edema
Patients receiving allogeneic transplant exhibited some form of edema (79%), hypervolemia, or documented weight increase (8%); all events were reported as mild or moderate.
Infection/Fever
Fifty-one percent (51%) of patients experienced one or more episodes of infection. Pneumonia was fatal in one patient (2%) and life-threatening in 3% of patients. Fever was reported in 80% of patients; it was mild or moderate in 78% and severe in 3%. Forty-six percent (46%) of patients experienced chills.
Cardiovascular
Mild or moderate tachycardia was reported in 44% of patients. In 7 patients (11%) it was first reported during busulfan administration. Other rhythm abnormalities, which were all mild or moderate, included arrhythmia (5%), atrial fibrillation (2%), ventricular extrasystoles (2%), and third degree heart block (2%). Mild or moderate thrombosis occurred in 33% of patients, and all episodes were associated with the central venous catheter. Hypertension was reported in 36% of patients and was Grade 3/4 in 7%. Hypotension occurred in 11% of patients and was Grade 3/4 in 3%. Mild vasodilation (flushing and hot flashes) was reported in 25% of patients. Other cardiovascular events included cardiomegaly (5%), mild ECG abnormality (2%), Grade 3/4 left-sided heart failure in one patient (2%), and moderate pericardial effusion (2%). These events were reported primarily in the post-cyclophosphamide phase.
Pulmonary
Mild or moderate dyspnea occurred in 25% of patients and was severe in 2%. One patient (2%) experienced severe hyperventilation; and in 2 (3%) additional patients it was mild or moderate. Mild rhinitis and mild or moderate cough were reported in 44% and 28% of patients, respectively. Mild epistaxis events were reported in 25%. Three patients (5%) on the allogeneic study developed documented alveolar hemorrhage. All required mechanical ventilatory support and all died. Non-specific interstitial fibrosis was found on wedge biopsies performed with video assisted thoracoscopy in one patient on the allogeneic study who subsequently died from respiratory failure on BMT Day +98. Other pulmonary events, reported as mild or moderate, included pharyngitis (18%), hiccup (18%), asthma (8%), atelectasis (2%), pleural effusion (3%), hypoxia (2%), hemoptysis (3%), and sinusitis (3%).
Neurologic
The most commonly reported adverse events of the central nervous system were insomnia (84%), anxiety (75%), dizziness (30%), and depression (23%). Severity was mild or moderate except for one patient (1%) who experienced severe insomnia. One patient (1%) developed a life-threatening cerebral hemorrhage and a coma as a terminal event following multi-organ failure after HVOD. Other events considered severe included delirium (2%), agitation (2%), and encephalopathy (2%). The overall incidence of confusion was 11%, and 5% of patients were reported to have experienced hallucinations. The patient who developed delirium and hallucination on the allogeneic study had onset of confusion at the completion of busulfan (busulfan) Injection. The overall incidence of lethargy in the allogeneic busulfan clinical trial was 7%, and somnolence was reported in 2%. One patient (2%) treated in an autologous transplantation study experienced a seizure while receiving cyclophosphamide, despite prophylactic treatment with phenytoin.
Renal
Creatinine was mildly or moderately elevated in 21% of patients. BUN was increased in 3% of patients and to a Grade 3/4 level in 2%. Seven percent of patients experienced dysuria, 15% oliguria, and 8% hematuria. There were 4 (7%) Grade 3/4 cases of hemorrhagic cystitis in the allogeneic clinical trial.
Skin
Rash (57%) and pruritus (28%) were reported; both conditions were predominantly mild. Alopecia was mild in 15% of patients and moderate in 2%. Mild vesicular rash was reported in 10% of patients and mild or moderate maculopapular rash in 8%. Vesiculo-bullous rash was reported in 10%, and exfoliative dermatitis in 5%. Erythema nodosum was reported in 2%, acne in 7%, and skin discoloration in 8%.
Metabolic
Hyperglycemia was observed in 67% of patients and Grade 3/4 hyperglycemia was reported in 15%. Hypomagnesemia was mild or moderate in 77% of patients; hypokalemia was mild or moderate in 62% and severe in 2%; hypocalcemia was mild or moderate in 46% and severe in 3%; hypophosphatemia was mild or moderate in 17%; and hyponatremia was reported in 2%.
Other
Other reported events included headache (mild or moderate 64%, severe 5%), abdominal pain (mild or moderate 69%, severe 3%), asthenia (mild or moderate 49%, severe 2%), unspecified pain (mild or moderate 43%, severe 2%), allergic reaction (mild or moderate 24%, severe 2%), injection site inflammation (mild or moderate 25%), injection site pain (mild or moderate 15%), chest pain (mild or moderate 26%), back pain (mild or moderate 23%), myalgia (mild or moderate 16%), arthralgia (mild or moderate 13%), and ear disorder in 3%.
Deaths
There were two deaths through BMT Day +28 in the allogeneic transplant setting. There were an additional six deaths BMT Day +29 through BMT Day +100 in the allogeneic transplant setting.
Postmarketing Experience
The following adverse reactions (reported as MedRA terms) have been identified during post-approval use of busulfan (busulfan) Injection: febrile neutropenia; tumor lysis syndrome; thrombotic micro-angiopathy (TMA); severe bacterial, viral (e.g., cytomegalovirus viraemia) and fungal infections; and sepsis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure.
Oral Busulfan Literature Review
A literature review identified four randomized, controlled trials that evaluated a high-dose oral busulfan-containing conditioning regimen for allogeneic bone marrow transplantation in the setting of CML. The safety outcomes reported in those trials are summarized in Table 4 below for a mixed population of hematological malignancies (AML, CML, and ALL).

Drug Interactions
Itraconazole decreases busulfan clearance by up to 25%, and may produce an AUC >1500 µM∙min in some patients. Fluconazole, and the 5-HT3 antiemetics odansetron (Zofran®) and granisetron (Kytril®) have all been used with busulfan.
Phenytoin increases the clearance of busulfan by 15% or more, possibly due to the induction of glutathione-S-transferase. Since the pharmacokinetics of busulfan were studied in patients treated with phenytoin, the clearance of busulfan at the recommended dose may be lower and exposure (AUC) higher in patients not treated with phenytoin.
Because busulfan is eliminated from the body via conjugation with glutathione, use of acetaminophen prior to (<72 hours) or concurrent with busulfan may result in reduced busulfan clearance based upon the known property of acetaminophen to decrease glutathione levels in the blood and tissues.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Busulfan may cause fetal harm when administered to a pregnant woman. Busulfan produced teratogenic changes in the offspring of mice, rats and rabbits when given during gestation. Malformations and anomalies included significant alterations in the musculoskeletal system, body weight gain, and size. In pregnant rats, busulfan produced sterility in both male and female offspring due to the absence of germinal cells in the testes and ovaries. The solvent, DMA, may also cause fetal harm when administered to a pregnant woman. In rats, DMA doses of 400 mg/kg/d (about 40% of the daily dose of DMA in the busulfan dose on a mg/m2 basis) given during organogenesis caused significant developmental anomalies. The most striking abnormalities included anasarca, cleft palate, vertebral anomalies, rib anomalies, and serious anomalies of the vessels of the heart. There are no adequate and well-controlled studies of either busulfan or DMA in pregnant women. If busulfan is used during pregnancy, or if the patient becomes pregnant while receiving busulfan, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Busulfan (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Busulfan (injection) during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for tumorgenicity shown for busulfan in human and animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The effectiveness of busulfan in the treatment of CML has not been specifically studied in pediatric patients.
Geriatic Use
Five of sixty-one patients treated in the busulfan clinical trial were over the age of 55 (range 57-64). All achieved myeloablation and engraftment.
Gender
djusting busulfan dosage based on gender has not been adequately studied.
Race
djusting busulfan dosage based on race has not been adequately studied.
Renal Impairment
Busulfan has not been studied in patients with renal impairment.
Hepatic Impairment
Busulfan has not been administered to patients with hepatic insufficiency.
Females of Reproductive Potential and Males
Ovarian suppression and amenorrhea commonly occur in premenopausal women undergoing chronic, low-dose busulfan therapy for chronic myelogenous leukemia. Busulfan depleted oocytes of female rats. Busulfan induced sterility in male rats and hamsters. Sterility, azoospermia and testicular atrophy have been reported in male patients.
The solvent DMA may also impair fertility. A DMA daily dose of 0.45 g/kg/d given to rats for nine days (equivalent to 44% of the daily dose of DMA contained in the recommended dose of busulfan on a mg/m2 basis) significantly decreased spermatogenesis in rats. A single sc dose of 2.2 g/kg (27% of the total DMA dose contained in busulfan on a mg/m2 basis) four days after insemination terminated pregnancy in 100% of tested hamsters.
Immunocompromised Patients
There is no FDA guidance one the use of Busulfan (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Busulfan (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Busulfan (injection) and IV administrations.
Overdosage
There is no known antidote to busulfan other than hematopoietic progenitor cell transplantation. In the absence of hematopoietic progenitor cell transplantation, the recommended dosage for busulfan would constitute an overdose of busulfan. The principal toxic effect is profound bone marrow hypoplasia/aplasia and pancytopenia, but the central nervous system, liver, lungs, and gastrointestinal tract may be affected. The hematologic status should be closely monitored and vigorous supportive measures instituted as medically indicated. Survival after a single 140 mg dose of Myleran® Tablets in an 18 kg, 4‑year old child has been reported. Inadvertent administration of a greater than normal dose of oral busulfan (2.1 mg/kg; total dose of 23.3 mg/kg) occurred in a 2‑year old child prior to a scheduled bone marrow transplant without sequelae. An acute dose of 2.4 g was fatal in a 10‑year old boy. There is one report that busulfan is dialyzable, thus dialysis should be considered in the case of overdose. Busulfan is metabolized by conjugation with glutathione, thus administration of glutathione may be considered.
Pharmacology
Mechanism of Action
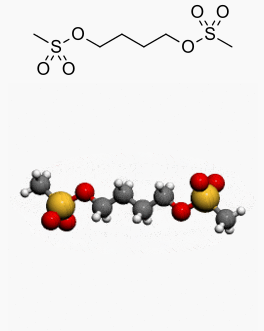
Busulfan is a bifunctional alkylating agent in which two labile methanesulfonate groups are attached to opposite ends of a four‑carbon alkyl chain. In aqueous media, busulfan hydrolyzes to release the methanesulfonate groups. This produces reactive carbonium ions that can alkylate DNA. DNA damage is thought to be responsible for much of the cytotoxicity of busulfan.
Structure
Busulfan is known chemically as 1,4-butanediol dimethanesulfonate and has the following structural formula: CH3SO2O(CH2)4OSO2CH3
Pharmacodynamics
There is limited information regarding Busulfan (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
The pharmacokinetics of busulfan were studied in 59 patients participating in a prospective trial of a busulfan-cyclophosphamide preparatory regimen prior to allogeneic hematopoietic progenitor stem cell transplantation. Patients received 0.8 mg/kg busulfan every six hours, for a total of 16 doses over four days. Fifty-five of fifty-nine patients (93%) administered busulfan maintained AUC values below the target value (<1500 µM∙min).

Busulfan pharmacokinetics showed consistency between dose 9 and dose 13 as demonstrated by reproducibility of steady state Cmax and a low coefficient of variation for this parameter.
In a pharmacokinetic study of busulfan in 24 pediatric patients, the population pharmacokinetic (PPK) estimates of busulfan for clearance (CL) and volume of distribution (V) were determined. For actual body weight, PPK estimates of CL and V were 4.04 L/hr/20 kg (3.37 mL/min/kg; interpatient variability 23%); and 12.8 L/20 kg (0.64 L/kg; interpatient variability 11%).
Distribution, Metabolism, Excretion
Studies of distribution, metabolism, and elimination of busulfan have not been done; however, the literature on oral busulfan is relevant. Additionally, for modulating effects on pharmacodynamic parameters see DRUG INTERACTIONS.
Distribution
Busulfan achieves concentrations in the cerebrospinal fluid approximately equal to those in plasma. Irreversible binding to plasma elements, primarily albumin, has been estimated to be 32.4±2.2% which is consistent with the reactive electrophilic properties of busulfan.
Metabolism
Busulfan is predominantly metabolized by conjugation with glutathione, both spontaneously and by glutathione S-transferase (GST) catalysis. This conjugate undergoes further extensive oxidative metabolism in the liver.
Excretion
Following administration of 14C‑labeled busulfan to humans, approximately 30% of the radioactivity was excreted into the urine over 48 hours; negligible amounts were recovered in feces. The incomplete recovery of radioactivity may be due to the formation of long-lived metabolites or due to nonspecific alkylation of macromolecules.
Nonclinical Toxicology
Carcinogenicity and Mutagenicity
Busulfan is a mutagen and a clastogen. In in vitro tests it caused mutations in Salmonella typhimurium and Drosophila melanogaster. Chromosomal aberrations induced by busulfan have been reported in vivo (rats, mice, hamsters, and humans) and in vitro (rodent and human cells). The intravenous administration of busulfan (48 mg/kg given as biweekly doses of 12 mg/kg, or 30% of the total busulfan dose on a mg/m2 basis) has been shown to increase the incidence of thymic and ovarian tumors in mice. Four cases of acute leukemia occurred among 19 patients who became pancytopenic in a 243 patient study incorporating busulfan as adjuvant therapy following surgical resection of bronchogenic carcinoma. Clinical appearance of leukemia was observed 5-8 years following oral busulfan treatment. Busulfan is a presumed human carcinogen.
Clinical Studies
Documentation of the safety and efficacy of busulfan as a component of a conditioning regimen prior to allogeneic hematopoietic progenitor cell reconstitution is derived from two sources: i) analysis of a prospective clinical trial of busulfan that involved 61 patients diagnosed with various hematologic malignancies, and ii) the published reports of randomized, controlled trials that employed high-dose oral busulfan as a component of a conditioning regimen for transplantation, which were identified in a literature review of five established commercial databases.
The prospective trial was a single-arm, open-label study in 61 patients who received busulfan as part of a conditioning regimen for allogeneic hematopoietic stem cell transplantation. The study included patients with acute leukemia past first remission (first or subsequent relapse), with high-risk first remission, or with induction failure; chronic myelogenous leukemia (CML) in chronic phase, accelerated phase, or blast crisis; primary refractory or resistant relapsed Hodgkin's disease or non-Hodgkin's lymphoma; and myelodysplastic syndrome. Forty-eight percent of patients (29/61) were heavily pretreated, defined as having at least one of the following: prior radiation, ≥3 prior chemotherapeutic regimens, or prior hematopoietic stem cell transplant. Seventy-five percent of patients (46/61) were transplanted with active disease.
Patients received 16 busulfan doses of 0.8 mg/kg every 6 hours as a two‑hour infusion for 4 days, followed by cyclophosphamide 60 mg/kg once per day for two days (BuCy2 regimen). All patients received 100% of their scheduled busulfan regimen. No dose adjustments were made. After one rest day, allogeneic hematopoietic progenitor cells were infused. The efficacy parameters in this study were myeloablation (defined as one or more of the following: absolute neutrophil count [ANC] less than 0.5×109/L, absolute lymphocyte count [ALC] less than 0.1×109/L, thrombocytopenia defined as a platelet count less than 20,000/mm3 or a platelet transfusion requirement) and engraftment (ANC ≥0.5×109/L).
All patients (61/61) experienced myeloablation. The median time to neutropenia was 4 days. All evaluable patients (60/60) engrafted at a median of 13 days post-transplant (range 9 to 29 days); one patient was considered non-evaluable because he died of a fungal pneumonia 20 days after BMT and before engraftment occurred. All but 13 of the patients were treated with prophylactic G-CSF. Evidence of donor cell engraftment and chimerism was documented in all patients who had a chromosomal sex marker or leukemic marker (43/43), and no patient with chimeric evidence of allogeneic engraftment suffered a later loss of the allogeneic graft. There were no reports of graft failure in the overall study population. The median number of platelet transfusions per patient was 6, and the median number of red blood cell transfusions per patient was 4.
Twenty-three patients (38%) relapsed at a median of 183 days post-transplant (range 36 to 406 days). Sixty-two percent of patients (38/61) were free from disease with a median follow-up of 269 days post-transplant (range 20 to 583 days). Forty-three patients (70%) were alive with a median follow up of 288 days post-transplant (range 51 to 583 days). There were two deaths before BMT Day +28 and six additional patients died by BMT Day +100. Ten patients (16%) died after BMT Day +100, at a median of 199 days post-transplant (range 113 to 275 days).
Oral Busulfan Literature Review
Four publications of randomized, controlled trials that evaluated a high-dose oral busulfan-containing conditioning regimen (busulfan 4 mg/kg/d ×4 days + cyclophosphamide 60 mg/kg/d ×2 days) for allogeneic transplantation in the setting of CML were identified. Two of the studies (Clift and Devergie) had populations confined to CML in chronic phase that were randomized between conditioning with busulfan/cyclophosphamide (BU/CY) and cyclophosphamide/total body irradiation (CY/TBI). A total of 138 patients were treated with BU/CY in these studies. The populations of the two remaining studies (Ringden and Blume) included patients with CML, acute lymphoblastic leukemia (ALL), and acute myelogenous leukemia (AML). In the Nordic BMT Group study published by Ringden, et al., 57 patients had CML, and of those, 30 were treated with BU/CY. Patients with CML in chronic phase, accelerated phase, and blast crisis were eligible for this study. The participants with CML (34/122 patients) in a SWOG study published by Blume, et al., had disease beyond first chronic phase. Twenty of those CML patients were treated with BU/CY, and the TBI comparator arm utilized etoposide instead of cyclophosphamide.

How Supplied
- Busulfan injection 6 mg/mL, single-Use Vials
- Unit carton of eight vials
- NDC 59148-070-91
Storage
Store between 2°-8°C (36°-46°F)
Images
Drug Images
{{#ask: Page Name::Busulfan (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Busulfan (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Busulfan (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Busulfan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Busulfex [1]
- Myleran
Look-Alike Drug Names
There is limited information regarding Busulfan (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Busulfan (injection) |Label Name=Busulfan 6mg-ml.png
}}