Bismuth subnitrate
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Bismuth subnitrate is used as an over-the-counter (OTC) antacid and anti-diarrheic agent.[1] Bismuth subnitrate is a homeopathic medication and lacks evaluation from the Food and Drug Administration (FDA) regarding its safety or efficacy. However, the use of bismuth substrate as an active ingredient in OTC antacids is approved by the FDA. Common adverse effects include headache, loss of appetite, and blue-gray skin discoloration.
Adult Indications and Dosage
Indications for Bismuth subnitrate use:
- Palliative Cancer Treatment:
- Used to prevent renal damage resulting from anti-cancer chemotherapy.
- Aids in preventing bone marrow damage caused by radiation therapy.
- Gastrointestinal Disorders:
- Topical Use:
- Acts as an astringent agent in the treatment of:
- Wounds
- Burns
- Hemorrhoids
- Other anorectal disorders.
- Acts as an astringent agent in the treatment of:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium selenite in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium selenite in adult patients.
Pediatric Indications and Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium selenite in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium selenite in adult patients.
Safety and effectiveness in pediatric patients have not been established.
Contraindications
Bismuth subnitrate is relatively contraindicated in patients with renal impairment and pregnant patients.
Warnings
Adverse Reactions
Loss of appetite, headache, blue-gray discoloration of skin, or renal damage. It can also cause black spots on the tongue and a thin blue-black line along the gum margin.
Drug Interactions
There is limited information regarding drug interactions of Bismuth subnitrate.
Use in Specific Populations
Use of Bismuth subnitrate in pregnant population is relatively contraindicated, particularly in the third trimester.
Administration and Monitoring
Overdosage
Reported oral doses associated with moderate to potentially lethal toxicity in humans range from 0.5 to 5 grams per kilogram. Symptoms of bismuth toxicity include osteoarthropathy, nephropathy, colitis, gingivitis, stomatitis, and encephalopathy.
Pharmacology
Clinical Studies
Package and Label Display Panel
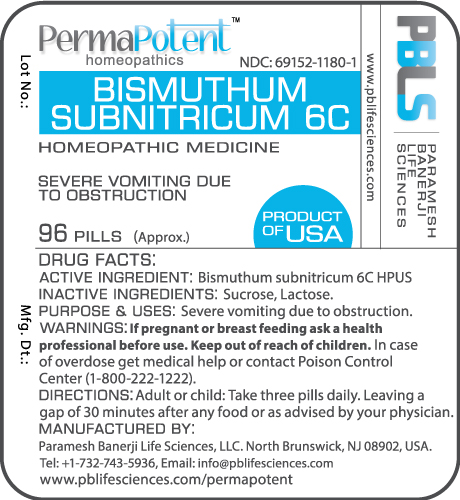
Brand Names
Bismithum Subnitriticum
Antimonite Belladona powder
Look-Alike Drug Names
There is limited information regarding Look-Alike Drug Names.
- ↑ Kondo Y, Himeno S, Satoh M, Naganuma A, Nishimura T, Imura N (2004). "Citrate enhances the protective effect of orally administered bismuth subnitrate against the nephrotoxicity of cis-diamminedichloroplatinum". Cancer Chemother Pharmacol. 53 (1): 33–8. doi:10.1007/s00280-003-0706-9. PMID 14530870.