Biocatalysis: Difference between revisions
No edit summary |
|||
| Line 33: | Line 33: | ||
In [[kinetic resolution]] of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the enantiomers into product at a greater [[reaction rate]] than the other enantiomer. | In [[kinetic resolution]] of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the enantiomers into product at a greater [[reaction rate]] than the other enantiomer. | ||
[[Image:Kineticresolution.gif | [[Image:Kineticresolution.gif|center|Scheme 1. Kinetic resolution]] | ||
The racemic mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology. The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower [[enantiomeric excess]]. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called '''dynamic resolution'''. | The racemic mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology. The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower [[enantiomeric excess]]. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called '''dynamic resolution'''. | ||
| Line 40: | Line 40: | ||
[[Image:Yeastreduction.gif | [[Image:Yeastreduction.gif|center|Scheme 2. Yeast reduction]] | ||
| Line 47: | Line 47: | ||
Another study demonstrates how racemic [[nicotine]] (mixture of S and R-enantiomers '''1''' in ''scheme 3'') can be deracemized in a [[One-pot synthesis|one-pot]] procedure involving a monoamine oxidase isolated from [[Aspergillus niger]] which is able to oxidize only the [[amine]] S-enantiomer to the [[imine]] '''2''' and involving an [[ammonia]] / [[borane]] [[reducing agent|reducing]] couple which can reduce the imine '''2''' back to the amine '''1''' <ref>{{cite journal| title=A Chemo-Enzymatic Route to Enantiomerically Pure Cyclic Tertiary Amines|author= Colin J. Dunsmore, Reuben Carr, Toni Fleming, and Nicholas J. Turner |journal= [[J. Am. Chem. Soc.]]| date=2006| volume=128|issue=7| pages= 2224 - 2225| doi=10.1021/ja058536d}}<br/></ref>. In this way the S-enantiomer will contineously be consumed by the emzyme while the R-enantiomer accumulates. It is even possible to [[stereoinversion|stereoinvert]] pure S to pure R. | Another study demonstrates how racemic [[nicotine]] (mixture of S and R-enantiomers '''1''' in ''scheme 3'') can be deracemized in a [[One-pot synthesis|one-pot]] procedure involving a monoamine oxidase isolated from [[Aspergillus niger]] which is able to oxidize only the [[amine]] S-enantiomer to the [[imine]] '''2''' and involving an [[ammonia]] / [[borane]] [[reducing agent|reducing]] couple which can reduce the imine '''2''' back to the amine '''1''' <ref>{{cite journal| title=A Chemo-Enzymatic Route to Enantiomerically Pure Cyclic Tertiary Amines|author= Colin J. Dunsmore, Reuben Carr, Toni Fleming, and Nicholas J. Turner |journal= [[J. Am. Chem. Soc.]]| date=2006| volume=128|issue=7| pages= 2224 - 2225| doi=10.1021/ja058536d}}<br/></ref>. In this way the S-enantiomer will contineously be consumed by the emzyme while the R-enantiomer accumulates. It is even possible to [[stereoinversion|stereoinvert]] pure S to pure R. | ||
[[Image:EnantiopuretertAmines.png|500px | [[Image:EnantiopuretertAmines.png|500px|center|Scheme 3. Enantiomerically Pure Cyclic Tertiary Amines]] | ||
==References== | ==References== | ||
Revision as of 21:07, 22 January 2009
|
WikiDoc Resources for Biocatalysis |
|
Articles |
|---|
|
Most recent articles on Biocatalysis Most cited articles on Biocatalysis |
|
Media |
|
Powerpoint slides on Biocatalysis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Biocatalysis at Clinical Trials.gov Clinical Trials on Biocatalysis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Biocatalysis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Biocatalysis Discussion groups on Biocatalysis Patient Handouts on Biocatalysis Directions to Hospitals Treating Biocatalysis Risk calculators and risk factors for Biocatalysis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Biocatalysis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Biocatalysis can be defined as the utilization of natural catalysts, called enzymes, to perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated or enzymes still residing inside living cells are employed for this task [1] [2] [3].
History
Biocatalysis underpins some of the oldest chemical transformations known to humans, for brewing predates recorded history. The oldest records of brewing are about 6000 years old and refer to the Sumerians.
The employment of enzymes and whole cells have been important for many industries for centuries. The most obvious usages have been in the food and drink businesses where the production of wine, beer, cheese etc. is dependent on the effects of the microorganisms.
More than one hundred years ago, biocatalysis was employed to do chemical transformations on non-natural man-made organic compounds, and the last 30 years have seen a substantial increase in the application of biocatalysis to produce fine chemicals, especially for the pharmaceutical industry.
Advantages of Biocatalysis
The key word for organic synthesis is selectivity which is necessary to obtain a high yield of a specific product. There are a large range of selective organic reactions available for most synthetic needs. However, there is still one area where organic chemists are struggling, and that is when chirality is involved, although considerable progress in chiral synthesis has been achieved in recent years.
Enzymes display three major types of selectivities:
- Chemoselectivity: Since the purpose of an enzyme is to act on a single type of functional group, other sensitive functionalities, which would normally react to a certain extent under chemical catalysis, survive. As a result, biocatalytic reactions tend to be "cleaner" and laborious purification of product(s) from impurities emerging through side-reactions can largely be omitted.
- Regioselectivity and Diastereoselectivity: Due to their complex three-dimensional structure, enzymes may distinguish between functional groups which are chemically situated in different regions of the substrate molecule.
- Enantioselectivity: Since almost all enzymes are made from L-amino acids, enzymes are chiral catalysts. As a consequence, any type of chirality present in the substrate molecule is "recognized" upon the formation of the enzyme-substrate complex. Thus a prochiral substrate may be transformed into an optically active product and both enantiomers of a racemic substrate may react at different rates.
These reasons, and especially the latter, are the major reasons why synthetic chemists have become interested in biocatalysis. This interest in turn is mainly due to the need to synthesise enantiopure compounds as chiral building blocks for drugs and agrochemicals.
Another important advantage of biocatalysts are that they are environmentally acceptable, being completely degraded in the environment. Furthermore the enzymes act under mild conditions, which minimizes problems of undesired side-reactions such as decomposition, isomerization, racemization and rearrangement, which often plague traditional methodology.
Asymmetric biocatalysis
The use of biocatalysis to obtain enantiopure compounds can be divided into two different methods;
- Kinetic resolution of a racemic mixture
- Biocatalysed asymmetric synthesis
In kinetic resolution of a racemic mixture, the presence of a chiral object (the enzyme) converts one of the enantiomers into product at a greater reaction rate than the other enantiomer.
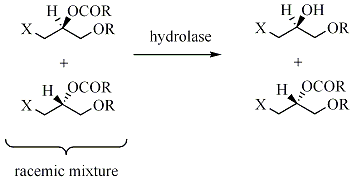
The racemic mixture has now been transformed into a mixture of two different compounds, making them separable by normal methodology. The maximum yield in such kinetic resolutions is 50%, since a yield of more than 50% means that some of wrong isomer also has reacted, giving a lower enantiomeric excess. Such reactions must therefore be terminated before equilibrium is reached. If it is possible to perform such resolutions under conditions where the two substrate- enantiomers are racemizing continuously, all substrate may in theory be converted into enantiopure product. This is called dynamic resolution.
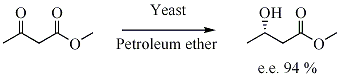
In biocatalysed asymmetric synthesis, a non-chiral unit becomes chiral in such a way that the different possible stereoismers are formed in different quantities. The chirality is introduced into the substrate by influence of enzyme, which is chiral. Yeast is a biocatalyst for the enantioselective reduction of ketones.
The biocatalytic Baeyer-Villiger oxidation is another example of a biocatalytic reaction. In one study a specially designed mutant of Candida antarctica was found to be an effective catalyst for the Michael addition of acrolein with acetylacetone at 20°C in absence of additional solvent [4].
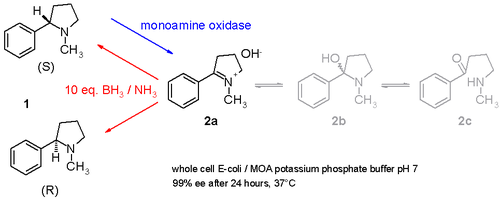
Another study demonstrates how racemic nicotine (mixture of S and R-enantiomers 1 in scheme 3) can be deracemized in a one-pot procedure involving a monoamine oxidase isolated from Aspergillus niger which is able to oxidize only the amine S-enantiomer to the imine 2 and involving an ammonia / borane reducing couple which can reduce the imine 2 back to the amine 1 [5]. In this way the S-enantiomer will contineously be consumed by the emzyme while the R-enantiomer accumulates. It is even possible to stereoinvert pure S to pure R.
References
- ↑ Anthonsen, T. Reactions Catalyzed by Enzymes. In Applied Biocatalysis, 2. Ed. ; Adlercreutz. P.; #Straathof, A. J. J. Eds.; Harwood Academic Publishers: UK, 1999; pp 18-53
- ↑ Faber, K. Biotransformations in Organic Chemistry, 4th ed., Springer-Verlag, Berlin 2000.
- ↑ Jayasinghe L. Y., Smallridge A. J., and Trewhella M. A. (1993). "The yeast mediated reduction of ethyl acetoacetate in petroleum ether". Tetrahedron Letters. 34 (24): 3949. doi:10.1016/S0040-4039(00)79272-0.
- ↑ Maria Svedendahl, Karl Hult, and Per Berglund (2005). "Fast Carbon-Carbon Bond Formation by a Promiscuous Lipase". J. Am. Chem. Soc. 127 (51): 17988–17989. doi:10.1021/ja056660r.
- ↑ Colin J. Dunsmore, Reuben Carr, Toni Fleming, and Nicholas J. Turner (2006). "A Chemo-Enzymatic Route to Enantiomerically Pure Cyclic Tertiary Amines". J. Am. Chem. Soc. 128 (7): 2224–2225. doi:10.1021/ja058536d.