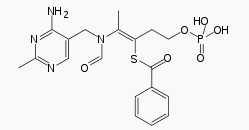
Benfotiamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Milgamma |
| Synonyms | S-Benzoylthiamine O-monophosphate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H23N4O6PS |
| Molar mass | 466.448 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Benfotiamine |
|
Articles |
|---|
|
Most recent articles on Benfotiamine Most cited articles on Benfotiamine |
|
Media |
|
Powerpoint slides on Benfotiamine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Benfotiamine at Clinical Trials.gov Clinical Trials on Benfotiamine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Benfotiamine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Benfotiamine Discussion groups on Benfotiamine Patient Handouts on Benfotiamine Directions to Hospitals Treating Benfotiamine Risk calculators and risk factors for Benfotiamine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Benfotiamine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Benfotiamine (rINN, or S-benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine (vitamin B1).
It has been licensed for use in Germany since 1993 under the trade name Milgamma. (Combinations with pyridoxine or cyanocobalamin are also sold under this name.) It is prescribed there for treating sciatica and other painful nerve conditions.[1]
It is marketed as a medicine and/or dietary supplement, depending on the respective Regulatory Authority.[citation needed]
Uses
Benfotiamine is primarily marketed as an antioxidant dietary supplement. In a clinical study with six patients, benfotiamine lowered AGE by 40%.[2]
Benfotiamine may be useful for the treatment of diabetic retinopathy, neuropathy, and nephropathy however "Most of the effects attributed to benfotiamine are extrapolated from in vitro and animal studies. Unfortunately apparent evidences from human studies are scarce and especially endpoint studies are missing. Therefore additional clinical studies are mandatory to explore the therapeutic potential of benfotiamine in both diabetic and non-diabetic pathological conditions".[3] It is thought that treatment with benfotiamine leads to increased intracellular thiamine diphosphate levels,[3] a cofactor of transketolase. This enzyme directs advanced glycation and lipoxidation end products (AGE's, ALE's) substrates to the pentose phosphate pathway, thus reducing tissue AGEs.[4][5][6][7][8]
Pharmacology
After absorption, benfotiamine can be dephosphorylated by cells bearing an ecto-alkaline phosphatase to the lipid-soluble S-benzoylthiamine.[9] Benfotiamine should not be confused with allithiamine, a naturally occurring thiamine disulfide derivative with a distinct pharmacological profile.[10]
See also
References
- ↑ "BBC news story: Back pain drug 'may aid diabetics'". BBC News. 18 February 2003.
- ↑ J Lin, A Alt, J Liersch, RG Bretzel, M Brownlee (May 2000). "Benfotiamine Inhibits Intracellular Formation of Advanced Glycation End Products in vivo". Diabetes. 49 (Suppl1) (A143): 583.
- ↑ 3.0 3.1 Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (2010). "The multifaceted therapeutic potential of benfotiamine". Pharmacol Res. 61 (6): 482–8. doi:10.1016/j.phrs.2010.02.008. PMID 20188835.
- ↑ Since AGEs are the actual agents productive of diabetic complications, in theory, if diabetic patients could block the action of AGEs completely by benfotiamine, strict blood sugar control, with its disruption of lifestyle and risks to health and life by severe hypoglycemic episodes, could be avoided, with revolutionary implications for the treatment of diabetes. Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9(3):294-299
- ↑ Stirban A, Negrean M, Stratmann B; et al. (2007). "Adiponectin decreases postprandially following a heat-processed meal in individuals with type 2 diabetes: an effect prevented by benfotiamine and cooking method". Diabetes Care. 30 (10): 2514–6. doi:10.2337/dc07-0302. PMID 17630265.
- ↑ Stracke H, Hammes HP, Werkmann D; et al. (2001). "Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats". Exp. Clin. Endocrinol. Diabetes. 109 (6): 330–6. doi:10.1055/s-2001-17399. PMID 11571671.
- ↑ Stirban A, Negrean M, Stratmann B; et al. (2006). "Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes". Diabetes Care. 29 (9): 2064–71. doi:10.2337/dc06-0531. PMID 16936154.
- ↑ Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ (2003). "Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine". Diabetes. 52 (8): 2110–20. doi:10.2337/diabetes.52.8.2110. PMID 12882930.
- ↑ Yamazaki M (1968), Studies on the absorption of S-benzoylthiamine O-monophosphate : (I) Metabolism in tissue homogenates. Vitamins 38 (1) 12–20.
- ↑ M.L. Volvert, S. Seyen, M. Piette, B. Evrard, M. Gangolf, J.C. Plumier and L. Bettendorff (2008) Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacology 8: 10. http://dx.doi.org/10.1186/1471-2210-8-10
External links
Template:Vitamins
Template:Neuropathic pain and fibromyalgia pharmacotherapies
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from April 2013
- Articles with invalid date parameter in template
- Drug
- Amines
- Pyrimidines
- Nitriles
- Organophosphates
- Thioesters
- Vitamin B1