Belimumab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=active autoantibody-positive [[systemic lupus erythematosus]] | |indication=active autoantibody-positive [[systemic lupus erythematosus]] | ||
|adverseReactions=Diarrhea, nausea, infectious disease, infusion reaction, nasopharyngitis, fever | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 14: | Line 15: | ||
|contraindications=*Belimumab is contraindicated in patients who have had [[anaphylaxis]] with belimumab. | |contraindications=*Belimumab is contraindicated in patients who have had [[anaphylaxis]] with belimumab. | ||
|warnings=====Mortality==== | |warnings=====Mortality==== | ||
*There were more deaths reported with belimumab than with [[placebo]] during the controlled period of the [[clinical trials]]. | *There were more [[deaths]] reported with belimumab than with [[placebo]] during the controlled period of the [[clinical trials]]. | ||
*Out of 2,133 patients in 3 [[clinical trials]], a total of 14 deaths occurred during the placebo-controlled, double-blind treatment periods: 3/675 (0.4%), 5/673 (0.7%), 0/111 (0%), and 6/674 (0.9%) deaths in the groups receiving [[placebo]], belimumab 1 mg/kg, belimumab 4 mg/kg, and belimumab 10 mg/kg, respectively. | *Out of 2,133 patients in 3 [[clinical trials]], a total of 14 deaths occurred during the placebo-controlled, double-blind treatment periods: 3/675 (0.4%), 5/673 (0.7%), 0/111 (0%), and 6/674 (0.9%) deaths in the groups receiving [[placebo]], belimumab 1 mg/kg, belimumab 4 mg/kg, and belimumab 10 mg/kg, respectively. | ||
*No single cause of death predominated. | *No single cause of death predominated. | ||
| Line 20: | Line 21: | ||
====Serious Infections==== | ====Serious Infections==== | ||
*Serious and sometimes fatal infections have been reported in patients receiving immunosuppressive agents, including belimumab. | *Serious and sometimes fatal [[infections]] have been reported in patients receiving [[immunosuppressive]] agents, including belimumab. | ||
*Physicians should exercise caution when considering the use of belimumab in patients with chronic infections. | *Physicians should exercise caution when considering the use of belimumab in patients with chronic infections. | ||
*Patients receiving any therapy for chronic infection should not begin therapy with belimumab. | *Patients receiving any therapy for chronic infection should not begin therapy with belimumab. | ||
Revision as of 16:11, 29 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Belimumab is a monoclonal antibody that is FDA approved for the treatment of active autoantibody-positive systemic lupus erythematosus. Common adverse reactions include Diarrhea, nausea, infectious disease, infusion reaction, nasopharyngitis, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Belimumab FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belimumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belimumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Belimumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belimumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belimumab in pediatric patients.
Contraindications
- Belimumab is contraindicated in patients who have had anaphylaxis with belimumab.
Warnings
Mortality
- There were more deaths reported with belimumab than with placebo during the controlled period of the clinical trials.
- Out of 2,133 patients in 3 clinical trials, a total of 14 deaths occurred during the placebo-controlled, double-blind treatment periods: 3/675 (0.4%), 5/673 (0.7%), 0/111 (0%), and 6/674 (0.9%) deaths in the groups receiving placebo, belimumab 1 mg/kg, belimumab 4 mg/kg, and belimumab 10 mg/kg, respectively.
- No single cause of death predominated.
- Etiologies included infection, cardiovascular disease, and suicide.
Serious Infections
- Serious and sometimes fatal infections have been reported in patients receiving immunosuppressive agents, including belimumab.
- Physicians should exercise caution when considering the use of belimumab in patients with chronic infections.
- Patients receiving any therapy for chronic infection should not begin therapy with belimumab.
- Consider interrupting therapy with belimumab in patients who develop a new infection while undergoing treatment with belimumab and monitor these patients closely.
- In the controlled clinical trials, the overall incidence of infections was 71% in patients treated with belimumab compared with 67% in patients who received placebo. *The most frequent infections (>5% of patients receiving belimumab) were upper respiratory tract infection, urinary tract infection, nasopharyngitis, sinusitis, bronchitis, and influenza. Serious infections occurred in 6.0% of patients treated with belimumab and in 5.2% of patients who received placebo.
- The most frequent serious infections included pneumonia, urinary tract infection, cellulitis, and bronchitis.
- Infections leading to discontinuation of treatment occurred in 0.7% of patients receiving belimumab and 1.0% of patients receiving placebo.
- Infections resulting in death occurred in 0.3% (4/1,458) of patients treated with belimumab and in 0.1% (1/675) of patients receiving placebo.
Progressive Multifocal Leukoencephalopathy (PML):
- Cases of JC virus-associated PML resulting in neurological deficits, including fatal cases, have been reported in patients with SLE receiving immunosuppressants, including belimumab.
- Risk factors for PML include treatment with immunosuppressant therapies and impairment of immune function.
- Consider the diagnosis of PML in any patient presenting with new-onset or deteriorating neurological signs and symptoms and consult with a neurologist or other appropriate specialist as clinically indicated.
- In patients with confirmed PML, consider stopping immunosuppressant therapy, including belimumab.
Malignancy
- The impact of treatment with belimumab on the development of malignancies is not known. *In the controlled clinical trials, malignancies (including non-melanoma skin cancers) were reported in 0.4% of patients receiving belimumab and 0.4% of patients receiving placebo.
- In the controlled clinical trials, malignancies, excluding non-melanoma skin cancers, were observed in 0.2% (3/1,458) and 0.3% (2/675) of patients receiving belimumab and placebo, respectively.
- The mechanism of action of belimumab could increase the risk for the development of malignancies.
Hypersensitivity Reactions, including Anaphylaxis
- Acute hypersensitivity reactions, including anaphylaxis and death, have been reported in association with belimumab.
- These events generally occurred within hours of the infusion; however, they may occur later.
- Non-acute hypersensitivity reactions including rash, nausea, fatigue, myalgia, headache, and facial edema, have been reported and typically occurred up to a week following the most recent infusion.
- Hypersensitivity, including serious reactions, has occurred in patients who have previously tolerated infusions of belimumab.
- Limited data suggest that patients with a history of multiple drug allergies or significant hypersensitivity may be at increased risk. In the controlled clinical trials, hypersensitivity reactions (occurring on the same day of infusion) were reported in 13% (191/1,458) of patients receiving belimumab and 11% (76/675) of patients receiving placebo.
- Anaphylaxis was observed in 0.6% (9/1,458) of patients receiving belimumab and 0.4% (3/675) of patients receiving placebo. Manifestations included hypotension, angioedema, urticaria or other rash, pruritus, and dyspnea.
- Due to overlap in signs and symptoms, it was not possible to distinguish between hypersensitivity reactions and infusion reactions in all cases.
- Some patients (13%) received premedication, which may have mitigated or masked a hypersensitivity response; however, there is insufficient evidence to determine whether premedication diminishes the frequency or severity of hypersensitivity reactions.
- Belimumab should be administered by healthcare providers prepared to manage anaphylaxis.
- In the event of a serious reaction, administration of belimumab must be discontinued immediately and appropriate medical therapy administered. Patients should be monitored during and for an appropriate period of time after administration of belimumab.
- Patients should be informed of the signs and symptoms of an acute hypersensitivity reaction and be instructed to seek immediate medical care should a reaction occur.
Infusion Reactions
- In the controlled clinical trials, adverse events associated with the infusion (occurring on the same day of the infusion) were reported in 17% (251/1,458) of patients receiving belimumab and 15% (99/675) of patients receiving placebo.
- Serious infusion reactions (excluding hypersensitivity reactions) were reported in 0.5% of patients receiving belimumab and 0.4% of patients receiving placebo and included bradycardia, myalgia, headache, rash, urticaria, and hypotension. *The most common infusion reactions (≥3% of patients receiving belimumab) were headache, nausea, and skin reactions.
- Due to overlap in signs and symptoms, it was not possible to distinguish between hypersensitivity reactions and infusion reactions in all cases.
- Some patients (13%) received premedication, which may have mitigated or masked an infusion reaction; however, there is insufficient evidence to determine whether premedication diminishes the frequency or severity of infusion reactions.
- Belimumab should be administered by healthcare providers prepared to manage infusion reactions.
- The infusion rate may be slowed or interrupted if the patient develops an infusion reaction.
- Healthcare providers should be aware of the risk of hypersensitivity reactions, which may present as infusion reactions, and monitor patients closely.
Depression
- In the controlled clinical trials, psychiatric events were reported more frequently with belimumab (16%) than with placebo (12%), related primarily to depression-related events (6.3% belimumab and 4.7% placebo), insomnia (6.0% belimumab and 5.3% placebo), and anxiety (3.9% belimumab and 2.8% placebo).
- Serious psychiatric events were reported in 0.8% of patients receiving belimumab (0.6% and 1.2% with 1 and 10 mg/kg, respectively) and 0.4% of patients receiving placebo. *Serious depression was reported in 0.4% (6/1,458) of patients receiving belimumab and 0.1% (1/675) of patients receiving placebo.
- Two suicides (0.1%) were reported in patients receiving belimumab.
- The majority of patients who reported serious depression or suicidal behavior had a history of depression or other serious psychiatric disorders and most were receiving psychoactive medications.
- It is unknown if treatment with belimumab is associated with increased risk for these events.
- Patients receiving belimumab should be instructed to contact their healthcare provider if they experience new or worsening depression, suicidal thoughts, or other mood changes.
Immunization
- Live vaccines should not be given for 30 days before or concurrently with belimumab as clinical safety has not been established.
- No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving belimumab or the effect of belimumab on new immunizations.
- Because of its mechanism of action, belimumab may interfere with the response to immunizations.
Concomitant Use with Other Biologic Therapies or Intravenous Cyclophosphamide
- Belimumab has not been studied in combination with other biologic therapies, including B-cell targeted therapies, or intravenous cyclophosphamide. Therefore, use of belimumab is not recommended in combination with biologic therapies or intravenous cyclophosphamide.
Adverse Reactions
Clinical Trials Experience
The data described below reflect exposure to belimumab plus standard of care compared with placebo plus standard of care in 2,133 patients in 3 controlled trials. Patients received belimumab at doses of 1 mg/kg (N = 673), 4 mg/kg (N = 111; Trial 1 only), or 10 mg/kg (N = 674) or placebo (N = 675) intravenously over a 1-hour period on Days 0, 14, 28, and then every 28 days. In 2 of the trials (Trial 1 and Trial 3), treatment was given for 48 weeks, while in the other trial (Trial 2) treatment was given for 72 weeks. Because there was no apparent dose-related increase in the majority of adverse events observed with belimumab, the safety data summarized below are presented for the 3 doses pooled, unless otherwise indicated; the adverse reaction table displays the results for the recommended dose of 10 mg/kg compared with placebo.
The population had a mean age of 39 (range: 18 to 75), 94% were female, and 52% were Caucasian. In these trials, 93% of patients treated with belimumab reported an adverse reaction compared with 92% treated with placebo.
The most common serious adverse reactions were serious infections (6.0% and 5.2% in the groups receiving belimumab and placebo, respectively).
The most commonly-reported adverse reactions, occurring in ≥5% of patients in clinical trials were nausea, diarrhea, pyrexia, nasopharyngitis, bronchitis, insomnia, pain in extremity, depression, migraine, and pharyngitis.
The proportion of patients who discontinued treatment due to any adverse reaction during the controlled clinical trials was 6.2% for patients receiving belimumab and 7.1% for patients receiving placebo. The most common adverse reactions resulting in discontinuation of treatment (≥1% of patients receiving belimumab or placebo) were infusion reactions (1.6% belimumab and 0.9% placebo), lupus nephritis (0.7% belimumab and 1.2% placebo), and infections (0.7% belimumab and 1.0% placebo).
TABLE 1 lists adverse reactions, regardless of causality, occurring in at least 3% of patients with SLE who received belimumab 10 mg/kg and at an incidence at least 1% greater than that observed with placebo in the 3 controlled studies.

Immunogenicity
In Trials 2 and 3, anti-belimumab antibodies were detected in 4 of 563 (0.7%) patients receiving belimumab 10 mg/kg and in 27 of 559 (4.8%) patients receiving belimumab 1 mg/kg. The reported frequency for the group receiving 10 mg/kg may underestimate the actual frequency due to lower assay sensitivity in the presence of high drug concentrations. Neutralizing antibodies were detected in 3 patients receiving belimumab 1 mg/kg. Three patients with anti-belimumab antibodies experienced mild infusion reactions of nausea, erythematous rash, pruritus, eyelid edema, headache, and dyspnea; none of the reactions was life-threatening. The clinical relevance of the presence of anti-belimumab antibodies is not known.
The data reflect the percentage of patients whose test results were positive for antibodies to belimumab in specific assays. The observed incidence of antibody positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to belimumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of belimumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Fatal anaphylaxis
Drug Interactions
- Formal drug interaction studies have not been performed with belimumab.
- In clinical trials of patients with SLE, belimumab was administered concomitantly with other drugs, including corticosteroids, antimalarials, immunomodulatory and immunosuppressive agents (including azathioprine, methotrexate, and mycophenolate), angiotensin pathway antihypertensives, HMG-CoA reductase inhibitors (statins), and NSAIDs without evidence of a clinically meaningful effect of these concomitant medications on belimumab pharmacokinetics.
- The effect of belimumab on the pharmacokinetics of other drugs has not been evaluated
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled clinical studies using belimumab in pregnant women. Immunoglobulin G (IgG) antibodies, including belimumab, can cross the placenta. Because animal reproduction studies are not always predictive of human response, belimumab should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Women of childbearing potential should use adequate contraception during treatment with belimumab and for at least 4 months after the final treatment.
Nonclinical reproductive studies have been performed in pregnant cynomolgus monkeys receiving belimumab at doses of 0, 5, and 150 mg/kg by intravenous infusion (the high dose was approximately 9 times the anticipated maximum human exposure) every 2 weeks from gestation day 20 to 150. Belimumab was shown to cross the placenta. Belimumab was not associated with direct or indirect teratogenicity under the conditions tested. Fetal deaths were observed in 14%, 24%, and 15% of pregnant females in the 0, 5 and 150 mg/kg groups, respectively. Infant deaths occurred with an incidence of 0%, 8%, and 5%. The cause of fetal and infant deaths is not known. The relevance of these findings to humans is not known. Other treatment-related findings were limited to the expected reversible reduction of B cells in both dams and infants and reversible reduction of immunoglobulin M (IgM) in infant monkeys. B-cell numbers recovered after the cessation of belimumab treatment by about 1 year post-partum in adult monkeys and by 3 months of age in infant monkeys. IgM levels in infants exposed to belimumab in utero recovered by 6 months of age.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Belimumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Belimumab during labor and delivery.
Nursing Mothers
It is not known whether belimumab is excreted in human milk or absorbed systemically after ingestion. However, belimumab was excreted into the milk of cynomolgus monkeys. Because maternal antibodies are excreted in human breast milk, a decision should be made whether to discontinue breastfeeding or to discontinue the drug, taking into account the importance of breastfeeding to the infant and the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of belimumab have not been established in children.
Geriatic Use
Clinical studies of belimumab did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Use with caution in elderly patients
Gender
There is no FDA guidance on the use of Belimumab with respect to specific gender populations.
Race
In Trial 2 and Trial 3, response rates for the primary endpoint were lower for black subjects receiving belimumab relative to black subjects receiving placebo. Use with caution in black/African-American patients.
Renal Impairment
There is no FDA guidance on the use of Belimumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Belimumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Belimumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Belimumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous infusion
- Belimumab should be administered by healthcare providers prepared to manage anaphylaxis.
- Belimumab should not be infused concomitantly in the same intravenous line with other agents.
- No physical or biochemical compatibility studies have been conducted to evaluate the coadministration of belimumab with other agents.
- The reconstituted solution of belimumab, if not used immediately, should be stored protected from direct sunlight and refrigerated at 2° to 8°C (36° to 46°F).
- Solutions of belimumab diluted in normal saline may be stored at 2° to 8°C (36° to 46°F) or room temperature. The total time from reconstitution of belimumab to completion of infusion should not exceed 8 hours.
Monitoring
- Healthcare providers should be aware of the risk of hypersensitivity reactions, which may present as infusion reactions, and monitor patients closely.
IV Compatibility
Belimumab is provided as a lyophilized powder in a single-use vial for intravenous infusion only and should be reconstituted and diluted by a healthcare professional using aseptic technique as follows:
Reconstitution Instructions
- Remove belimumab from the refrigerator and allow to stand 10 to 15 minutes for the vial to reach room temperature.
- Reconstitute the belimumab powder with Sterile Water for Injection, USP, as follows. The reconstituted solution will contain a concentration of 80 mg/mL belimumab.
- Reconstitute the 120-mg vial with 1.5 mL Sterile Water for Injection, USP.
- Reconstitute the 400-mg vial with 4.8 mL Sterile Water for Injection, USP.
- The stream of sterile water should be directed toward the side of the vial to minimize foaming.
- Gently swirl the vial for 60 seconds.
- Allow the vial to sit at room temperature during reconstitution, gently swirling the vial for 60 seconds every 5 minutes until the powder is dissolved.
- Do not shake.
- Reconstitution is typically complete within 10 to 15 minutes after the sterile water has been added, but it may take up to 30 minutes.
- Protect the reconstituted solution from sunlight.
- If a mechanical reconstitution device (swirler) is used to reconstitute belimumab, it should not exceed 500 rpm and the vial swirled for no longer than 30 minutes.
- Once reconstitution is complete, the solution should be opalescent and colorless to pale yellow, and without particles.
- Small air bubbles, however, are expected and acceptable.
Dilution Instructions
- Dextrose intravenous solutions are incompatible with belimumab.
- Belimumab should only be diluted in 0.9% Sodium Chloride Injection, USP.
- Dilute the reconstituted product to 250 mL in 0.9% Sodium Chloride Injection, USP (normal saline) for intravenous infusion.
- From a 250-mL infusion bag or bottle of normal saline, withdraw and discard a volume equal to the volume of the reconstituted solution of belimumab required for the patient’s dose. Then add the required volume of the reconstituted solution of belimumab into the infusion bag or bottle.
- Gently invert the bag or bottle to mix the solution. Any unused solution in the vials must be discarded.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Discard the solution if any particulate matter or discoloration is observed.
- The reconstituted solution of belimumab, if not used immediately, should be stored protected from direct sunlight and refrigerated at 2° to 8°C (36° to 46°F).
- Solutions of belimumab diluted in normal saline may be stored at 2° to 8°C (36° to 46°F) or room temperature.
- The total time from reconstitution of belimumab to completion of infusion should not exceed 8 hours.
- No incompatibilities between belimumab and polyvinylchloride or polyolefin bags have been observed.
Overdosage
- There is no clinical experience with overdosage of belimumab.
- Two doses of up to 20 mg/kg have been given by intravenous infusion to humans with no increase in incidence or severity of adverse reactions compared with doses of 1, 4, or 10 mg/kg.
Pharmacology
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Template:Infobox drug/mab source |
| Target | B-cell activating factor (BAFF, BLyS) |
| Clinical data | |
| Trade names | Benlysta |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611027 |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6714H10428O2102S52 |
| Molar mass | 151.8 kDa |
| | |
Mechanism of Action
Belimumab is a BLyS-specific inhibitor that blocks the binding of soluble BLyS, a B-cell survival factor, to its receptors on B cells. Belimumab does not bind B cells directly, but by binding BLyS, belimumab inhibits the survival of B cells, including autoreactive B cells, and reduces the differentiation of B cells into immunoglobulin-producing plasma cells.
Structure
Belimumab is a human IgG1λ monoclonal antibody specific for soluble human B lymphocyte stimulator protein (BLyS, also referred to as BAFF and TNFSF13B). Belimumab has a molecular weight of approximately 147 kDa. Belimumab is produced by recombinant DNA technology in a mammalian cell expression system.
Belimumab is supplied as a sterile, white to off-white, preservative-free, lyophilized powder for intravenous infusion. Upon reconstitution with Sterile Water for Injection, USP, each single-use vial delivers 80 mg/mL belimumab in 0.16 mg/mL citric acid, 0.4 mg/mL polysorbate 80, 2.7 mg/mL sodium citrate, and 80 mg/mL sucrose, with a pH of 6.5.
Pharmacodynamics
In Trial 1 and Trial 2 in which B cells were measured, treatment with belimumab significantly reduced circulating CD19+, CD20+, naïve, and activated B cells, plasmacytoid cells, and the SLE B-cell subset at Week 52. Reductions in naïve and the SLE B-cell subset were observed as early as Week 8 and were sustained to Week 52. Memory cells increased initially and slowly declined toward baseline levels by Week 52. The clinical relevance of these effects on B cells has not been established.
Treatment with belimumab led to reductions in IgG and anti-dsDNA, and increases in complement (C3 and C4). These changes were observed as early as Week 8 and were sustained through Week 52. The clinical relevance of normalizing these biomarkers has not been definitively established.
Pharmacokinetics
The pharmacokinetic parameters displayed in TABLE 2 are based on population parameter estimates which are specific to the 563 patients who received belimumab 10 mg/kg in Trials 2 and 3.
Drug Interactions: No formal drug interaction studies have been conducted with belimumab. Concomitant use of mycophenolate, azathioprine, methotrexate, antimalarials, NSAIDs, aspirin, and HMG-CoA reductase inhibitors did not significantly influence belimumab pharmacokinetics. Coadministration of steroids and angiotensin-converting enzyme (ACE) inhibitors resulted in an increase of systemic clearance of belimumab that was not clinically significant because the magnitude was well within the range of normal variability of clearance. The effect of belimumab on the pharmacokinetics of other drugs has not been evaluated.
Special Populations: The following information is based on the population pharmacokinetic analysis.
Age: Age did not significantly influence belimumab pharmacokinetics in the trial population, where the majority of subjects (70%) were aged between 18 and 45 years. No pharmacokinetic data are available in pediatric patients. Limited pharmacokinetic data are available for elderly patients as only 1.4% of the subjects included in the pharmacokinetic analysis were aged 65 years or older.
Gender: Gender did not significantly influence belimumab pharmacokinetics in the largely (94%) female trial population.
Race: Race did not significantly influence belimumab pharmacokinetics. The racial distribution was 53% white/Caucasian, 16% Asian, 16% Alaska native/American Indian, and 14% black/African-American.
Renal Impairment: No formal trials were conducted to examine the effects of renal impairment on the pharmacokinetics of belimumab. Belimumab has been studied in a limited number of patients with SLE and renal impairment (261 subjects with moderate renal impairment, creatinine clearance ≥30 and <60 mL/min; 14 subjects with severe renal impairment, creatinine clearance ≥15 and <30 mL/min). Although increases in creatinine clearance and proteinuria (>2 g/day) increased belimumab clearance, these effects were within the expected range of variability. Therefore, dosage adjustment in patients with renal impairment is not recommended.
Hepatic Impairment: No formal trials were conducted to examine the effects of hepatic impairment on the pharmacokinetics of belimumab. Baseline ALT and AST levels did not significantly influence belimumab pharmacokinetics.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of belimumab. The mutagenic potential of belimumab was not evaluated.
Effects on male and female fertility have not been directly evaluated in animal studies.
Clinical Studies
The safety and effectiveness of belimumab were evaluated in 3 randomized, double-blind, placebo-controlled trials involving 2,133 patients with SLE according to the American College of Rheumatology criteria (Trial 1, 2, and 3). Patients with severe active lupus nephritis and severe active CNS lupus were excluded. Patients were on a stable standard of care SLE treatment regimen comprising any of the following (alone or in combination): corticosteroids, antimalarials, NSAIDs, and immunosuppressives. Use of other biologics and intravenous cyclophosphamide were not permitted.
```Trial 1: belimumab 1 mg/kg, 4 mg/kg, 10 mg/kg
Trial 1 enrolled 449 patients and evaluated doses of 1, 4, and 10 mg/kg belimumab plus standard of care compared with placebo plus standard of care over 52 weeks in patients with SLE. Patients had to have a SELENA-SLEDAI score of ≥4 at baseline and a history of autoantibodies (anti-nuclear antibody [ANA] and/or anti-double-stranded DNA [anti-dsDNA]), but 28% of the population was autoantibody negative at baseline. The co-primary endpoints were percent change in SELENA-SLEDAI score at Week 24 and time to first flare over 52 weeks. No significant differences between any of the groups receiving belimumab and the group receiving placebo were observed. Exploratory analysis of this trial identified a subgroup of patients (72%), who were autoantibody positive, in whom belimumab appeared to offer benefit. The results of this trial informed the design of Trials 2 and 3 and led to the selection of a target population and indication that is limited to autoantibody-positive SLE patients.
Trials 2 and 3: belimumab 1 mg/kg and 10 mg/kg Trials 2 and 3 were randomized, double-blind, placebo-controlled trials in patients with SLE that were similar in design except duration - Trial 2 was 76 weeks duration and Trial 3 was 52 weeks duration. Eligible patients had active SLE disease, defined as a SELENA-SLEDAI score ≥6, and positive autoantibody test results at screening. Patients were excluded from the trial if they had ever received treatment with a B-cell targeted agent or if they were currently receiving other biologic agents. Intravenous cyclophosphamide was not permitted within the previous 6 months or during the trial. Trial 2 was conducted primarily in North America and Europe. Trial 3 was conducted in South America, Eastern Europe, Asia, and Australia.
Baseline concomitant medications included corticosteroids (Trial 2: 76%, Trial 3: 96%), immunosuppressives (Trial 2: 56%, Trial 3: 42%; including azathioprine, methotrexate and mycophenolate), and antimalarials (Trial 2: 63%, Trial 3: 67%). Most patients (>70%) were receiving 2 or more classes of SLE medications.
In Trial 2 and Trial 3, more than 50% of patients had 3 or more active organ systems at baseline. The most common active organ systems at baseline based on SELENA-SLEDAI were mucocutaneous (82% in both trials); immune (Trial 2: 74%, Trial 3: 85%); and musculoskeletal (Trial 2: 73%, Trial 3: 59%). Less than 16% of patients had some degree of renal activity and less than 7% of patients had activity in the vascular, cardio-respiratory, or CNS systems.
At screening, patients were stratified by disease severity based on their SELENA-SLEDAI score (≤9 vs. ≥10), proteinuria level (<2 g/24 hr vs. ≥2 g/24 hr), and race (African or Indigenous-American descent vs. other), and then randomly assigned to receive belimumab 1 mg/kg, belimumab 10 mg/kg, or placebo in addition to standard of care. The patients were administered trial medication intravenously over a 1-hour period on Days 0, 14, 28, and then every 28 days for 48 weeks in Trial 3 and for 72 weeks in Trial 2.
The primary efficacy endpoint was a composite endpoint (SLE Responder Index or SRI) that defined response as meeting each of the following criteria at Week 52 compared with baseline:
- ≥4-point reduction in the SELENA-SLEDAI score, and
- no new British Isles Lupus Assessment Group (BILAG) A organ domain score or 2 new BILAG B organ domain scores, and
- no worsening (<0.30-point increase) in Physician’s Global Assessment (PGA) score.
The SRI uses the SELENA-SLEDAI score as an objective measure of reduction in global disease activity; the BILAG index to ensure no significant worsening in any specific organ system; and the PGA to ensure that improvements in disease activity are not accompanied by worsening of the patient’s condition overall.
In both Trials 2 and 3, the proportion of SLE patients achieving an SRI response, as defined for the primary endpoint, was significantly higher in the group receiving belimumab 10 mg/kg than in the group receiving placebo. The effect on the SRI was not consistently significantly different for patients receiving belimumab 1 mg/kg relative to placebo in both trials. The 1 mg/kg dose is not recommended. The trends in comparisons between the treatment groups for the rates of response for the individual components of the endpoint were generally consistent with that of the SRI (Table 3). At Week 76 in Trial 2, the SRI response rate with belimumab 10 mg/kg was not significantly different from that of placebo (39% and 32%, respectively).
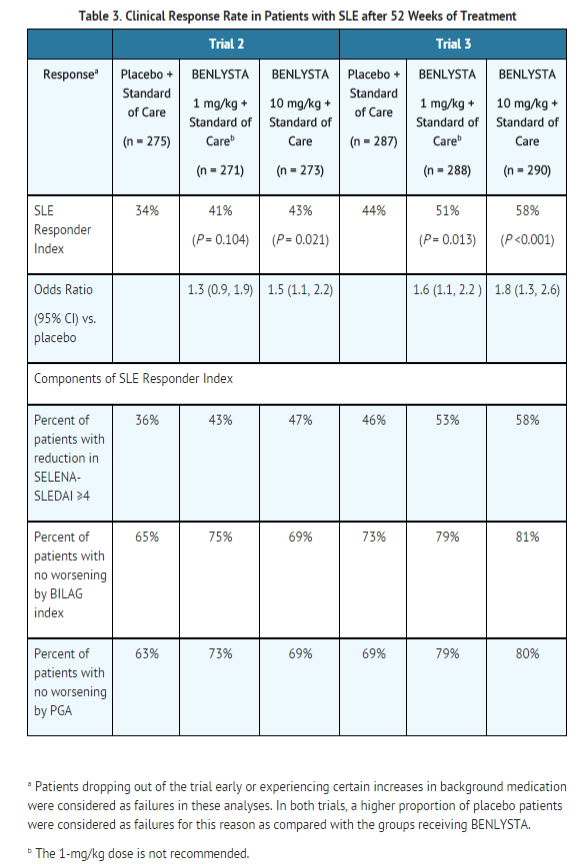
The reduction in disease activity seen in the SRI was related primarily to improvement in the most commonly involved organ systems namely, mucocutaneous, musculoskeletal, and immune.
Effect in Black/African-American Patients: Exploratory sub-group analyses of SRI response rate in patients of black race were performed. In Trial 2 and Trial 3 combined, the SRI response rate in black patients (N = 148) in groups receiving belimumab was less than that in the group receiving placebo (22/50 or 44% for placebo, 15/48 or 31% for belimumab 1 mg/kg, and 18/50 or 36% for belimumab 10 mg/kg). In Trial 1, black patients (N = 106) in the groups receiving belimumab did not appear to have a different response than the rest of the trial population. Although no definitive conclusions can be drawn from these subgroup analyses, caution should be used when considering treatment with belimumab in black/African-American SLE patients.
Effect on Concomitant Steroid Treatment: In Trial 2 and Trial 3, 46% and 69% of patients, respectively, were receiving prednisone at doses >7.5 mg/day at baseline. The proportion of patients able to reduce their average prednisone dose by at least 25% to ≤7.5 mg/day during Weeks 40 through 52 was not consistently significantly different for belimumab relative to placebo in both trials. In Trial 2, 17% of patients receiving belimumab 10 mg/kg and 19% of patients receiving belimumab 1 mg/kg achieved this level of steroid reduction compared with 13% of patients receiving placebo. In Trial 3, 19%, 21%, and 12% of patients receiving belimumab 10 mg/kg, belimumab 1 mg/kg, and placebo, respectively, achieved this level of steroid reduction.
Effect on Severe SLE Flares: The probability of experiencing a severe SLE flare, as defined by a modification of the SELENA Trial flare criteria which excluded severe flares triggered only by an increase of the SELENA-SLEDAI score to >12, was calculated for both Trials 2 and 3. The proportion of patients having at least 1 severe flare over 52 weeks was not consistently significantly different for belimumab relative to placebo in both trials. In Trial 2, 18% of patients receiving belimumab 10 mg/kg and 16% of patients receiving belimumab 1 mg/kg had a severe flare compared with 24% of patients receiving placebo. In Trial 3, 14%, 18%, and 23% of patients receiving belimumab 10 mg/kg, belimumab 1 mg/kg and placebo, respectively, had a severe flare.
How Supplied
- Belimumab is a sterile, preservative-free, lyophilized powder for reconstitution, dilution, and intravenous infusion provided in single-use glass vials with a rubber stopper (not made with natural rubber latex) and a flip-off seal.
- Each 5-mL vial contains 120 mg of belimumab.
- Each 20-mL vial contains 400 mg of belimumab.
Belimumab is supplied as follows:

Storage
- Store vials of belimumab refrigerated between 2° to 8°C (36° to 46°F).
- Vials should be protected from light and stored in the original carton until use.
- Do not freeze.
- Avoid exposure to heat.
- Do not use beyond the expiration date.
Images
Drug Images
{{#ask: Page Name::Belimumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Belimumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advice for the Patient
- Give patients the Medication Guide for belimumab and provide them an opportunity to read it prior to each treatment session.
- It is important that the patient’s overall health be assessed at each infusion visit and any questions resulting from the patient’s reading of the Medication Guide be discussed.
Mortality: Advise patients that more patients receiving belimumab in the main clinical trials died than did patients receiving placebo treatment
Serious Infections: Advise patients that belimumab may decrease their ability to fight infections. Ask patients if they have a history of chronic infections and if they are currently on any therapy for an infection. Instruct patients to tell their healthcare provider if they develop signs or symptoms of an infection.
Progressive Multifocal Leukoencephalopathy (PML): Advise patients to contact their healthcare professional if they experience new or worsening neurological symptoms such as memory loss, confusion, dizziness or loss of balance, difficulty talking or walking, or vision problems.
Hypersensitivity/Anaphylactic and Infusion Reactions: Educate patients on the signs and symptoms of hypersensitivity and infusion reactions, including wheezing, difficulty breathing, angioedema, rash, hypotension, bradycardia, and headache. Instruct patients to immediately tell their healthcare provider if they experience symptoms of an allergic reaction during or after the administration of belimumab. Inform patients to tell their healthcare provider about possible reactions that may include a combination of symptoms such as rash, nausea, fatigue, muscle aches, headache, and/or facial swelling and may occur after administration of belimumab.
Depression: Instruct patients to contact their healthcare provider if they experience new or worsening depression, suicidal thoughts or other mood changes.
Immunizations: Inform patients that they should not receive live vaccines while taking belimumab. Response to vaccinations could be impaired by belimumab.
Pregnancy and Nursing Mothers: Inform patients that belimumab has not been studied in pregnant women or nursing mothers so the effects of belimumab on pregnant women or nursing infants are not known. Instruct patients to tell their healthcare provider if they are pregnant, become pregnant, or are thinking about becoming pregnant. Encourage pregnant patients to enroll in the pregnancy registry for belimumab. Instruct patients to tell their healthcare provider if they plan to breastfeed their infant.
Precautions with Alcohol
Alcohol-Belimumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Benlysta[1]
Look-Alike Drug Names
There is limited information regarding Belimumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed KEGG identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Drugboxes which contain changes to verified fields
- Infobox drug tracked parameters
- Drugs that are a monoclonal antibody
