Beer-Lambert law
|
WikiDoc Resources for Beer-Lambert law |
|
Articles |
|---|
|
Most recent articles on Beer-Lambert law Most cited articles on Beer-Lambert law |
|
Media |
|
Powerpoint slides on Beer-Lambert law |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Beer-Lambert law at Clinical Trials.gov Trial results on Beer-Lambert law Clinical Trials on Beer-Lambert law at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Beer-Lambert law NICE Guidance on Beer-Lambert law
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Beer-Lambert law Discussion groups on Beer-Lambert law Patient Handouts on Beer-Lambert law Directions to Hospitals Treating Beer-Lambert law Risk calculators and risk factors for Beer-Lambert law
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Beer-Lambert law |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
In optics, the Beer–Lambert law, also known as Beer's law or the Lambert–Beer law or the Beer–Lambert–Bouguer law (in fact, most of the permutations of these three names appear somewhere in literature) is an empirical relationship that relates the absorption of light to the properties of the material through which the light is travelling.
Equations
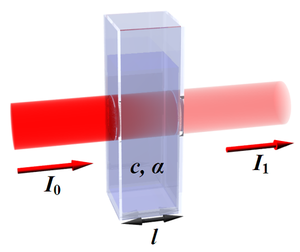
The law states that there is a logarithmic dependence between the transmission (or transmissivity), <math> T \, </math>, of light through a substance and the product of the absorption coefficient of the substance, <math>\alpha \,</math>, and the distance the light travels through the material (i.e. the path length), <math>l \,</math>. The absorption coefficient can, in turn, be written as a product of either a molar absorptivity of the absorber, <math>\epsilon \,</math>, and the concentration of absorbing species in the material, <math>c \,</math>, or an absorption cross section, <math>\sigma \,</math>, and the (number) density of absorbers, <math>N \,</math>.
For liquids, these relations are usually written as
- <math> T = {I_{1}\over I_{0}} = 10^{-\alpha\, l} = 10^{-\epsilon \, l \, c} </math>
whereas for gases, and in particular among physicists and for spectroscopy and spectrophotometry, they are normally written
- <math> T = {I_{1}\over I_{0}} = e^{-\alpha'\, l} = e^{-\sigma l N} </math>
where <math>I_0 \,</math> and <math>I_1 \,</math> are the intensity (or power) of the incident light and that after the material, respectively.
The transmission (or transmissivity) is expressed in terms of an absorbance which for liquids is defined as
- <math> A = -\log_{10} \left( \frac{I_1}{I_0} \right)</math>
whereas it is usually defined as
- <math> A' = -\ln \left( \frac{I_1}{I_0} \right)</math>
for gases.
This implies that the absorbance becomes linear with the concentration (or number density of absorbers) according to
- <math> A = \epsilon \, l\,c \, = \alpha \,l \,</math>
and
- <math> A' = \sigma \, l\,N \,= \alpha' \,l \,</math>
for the two cases, respectively.
Thus, if the path length and the molar absorptivity (or the absorption cross section) are known and the absorbance is measured, the concentration of the substance (or the number density of absorbers) can be deduced.
Although several of the expressions above often are used as Beer–Lambert law, the name should strictly speaking only by associated with the latter two. The reason is that historically, the Lambert law states that absorption is proportional to the light path length, whereas the Beer law states that absorption is proportional to the concentration of absorbing species in the material.[1]
If the concentration is expressed as a mole fraction i.e. a dimensionless fraction, the molar absorptivity (<math>\epsilon \,</math>) takes the same dimension as the absorption coefficient, i.e. reciprocal length (e.g. cm−1). However, if the concentration is expressed in moles per unit volume, the molar absorptivity (<math>\epsilon \,</math>) is used in L·mol−1·cm−1, or sometimes in converted units of mol−1 cm2.
Moreover, the absorption coefficient <math>\alpha' \,</math> can be expressed in term of the imaginary part of the refractive index, <math>\kappa \,</math> , and the wavelength of the light (in free space), <math>\lambda_{0} \,</math>, according to
- <math> \alpha' = \frac{4 \pi \kappa}{\lambda_{0}}</math>
In molecular absorption spectrometry, the absorption cross section σ is expressed in terms of a linestrength, S, and an (area-normalized) lineshape function, Φ. The frequency scale in molecular spectroscopy is often in cm-1, wherefore the lineshape function is expressed in units of 1/cm-1, which can look funny but is strictly correct. Since N is given as a number density in units of 1/cm3, the linestrength is often given in units of cm2cm-1/molecule. A typical linestrength in one of the vibrational overtone bands of smaller molecules, e.g. around 1.5 μm in CO or CO2, is around 10-23 cm2cm-1, although it can be larger for species with strong transitions, e.g. C2H2. The linestrengths of various transitions can be found in large databases, e.g. HITRAN. The lineshape function often takes a value around a few 1/cm-1, up to around 10/cm-1 under low pressure conditions, when the transition is Doppler broadened, and below this under atmospheric pressure conditions, when the transition is collision broadened. It has also become commonplace to express the linestrength in units of cm-2/atm since then the concentration is given in terms of a pressure in units of atm. A typical linestrength is then often in the order of 10-3 cm-2/atm. Under these conditions, the detectability of a given technique is often quoted in terms of ppm•m.
The fact that there are two commensurate definitions of absorbance (in base 10 or e) implies that the absorbance and the absorption coefficient for the cases with gases, <math> A' \, </math> and <math>\alpha' \,</math>, are ln 10 (approximately 2.3) times larger than the corresponding values for liquids, i.e. <math> A \, </math> and <math>\alpha \,</math>, respectively. Therefore, care must be taken when interpreting data that the correct form of the law is used.
The law tends to break down at very high concentrations, especially if the material is highly scattering. If the light is especially intense, nonlinear optical processes can also cause variances.
Derivation
Assume that particles may be described as having an absorption cross section (i.e. area), σ, perpendicular to the path of light through a solution, such that a photon of light is absorbed if it strikes the particle, and is transmitted if it does not.
Define z as an axis parallel to the direction that photons of light are moving, and A and dz as the area and thickness (along the z axis) of a 3-dimensional slab of space through which light is passing. We assume that dz is sufficiently small that one particle in the slab cannot obscure another particle in the slab when viewed along the z direction. The concentration of particles in the slab is represented by N.
It follows that the fraction of photons absorbed when passing through this slab is equal to the total opaque area of the particles in the slab, σ A N dz, divided by the area of the slab A, which yields σ N dz. Expressing the number of photons absorbed by the slab as dIz, and the total number of photons incident on the slab as Iz, the fraction of photons absorbed by the slab is given by
- <math> \frac{dI_z}{I_z} = - \sigma N\,dz .</math>
The solution to this simple differential equation is obtained by integrating both sides to obtain Iz as a function of z
- <math> \ln(I_z) = - \sigma N z + C . \,</math>
The difference of intensity for a slab of real thickness ℓ is I0 at z = 0, and I1 at z = ℓ. Using the previous equation, the difference in intensity can be written as,
- <math>\ln(I_0) - \ln(I_\ell) = (- \sigma 0 N + C) - ( - \sigma \, l\,N \,+ C) = \sigma \, l\,N \,</math>
rearranging and exponentiating yields,
- <math>\ T = \frac{I_1}{I_0} = e ^ {- \sigma \,l\, N} = e ^ {- \alpha' \,l} .</math>
This implies that
- <math> A' = - \ln\left( \frac{I_1}{I_0} \right) = \alpha' \, l\,= \sigma \, l\,N \,</math>
and
- <math> A = - \log_{10}\left( \frac{I_1}{I_0} \right) = \alpha' / 2.30\, l\,= \alpha \, l\,= \epsilon \, l\,c \,</math>
It is instructive to consider the consequences of error in an assumption that is implicit in this derivation, namely that every absorbing particle behaves independently with respect to the light. Error is introduced when particles interact by lying along the same optical path such that some particles are in the shadow of others. The assumption approaches accuracy only in very dilute solutions, and it becomes increasingly inaccurate with increasingly concentrated solutions or long optical paths.
In practice, the accuracy of the assumption is better than the accuracy of most spectroscopic measurements up to an absorbance of 1 (or : <math>{I_1} / {I_0} = 0.1) </math> and to a good approximation, measurements of absorbance in this range are linearly related to the concentration of absorbing substances in solution. At higher absorbances, concentrations will be underestimated due to this shadow effect unless one employs a nonlinear relationship between absorbance and concentration.
Prerequisites
There are at least five conditions that need to be fulfilled in order for Beer’s law to be valid. These are:
- The absorbers must act independently of each other;
- The absorbing medium must be homogeneously distributed in the interaction volume and must not scatter the radiation;
- The incident radiation must consist of parallel rays, each traversing the same length in the absorbing medium;
- The incident radiation should preferably be monochromatic, or have at least a width that is more narrow than the absorbing transition; and
- The incident flux must not influence the atoms or molecules; it should only act as a non-invasive probe of the species under study. In particular, this implies that the light should not cause optical saturation or optical pumping, since such effects will deplete the lower level and possibly give rise to stimulated emission.
If any of these conditions is not fulfilled, there will be deviations from Beer’s law.
Chemical analysis
Beer's law can be applied to the analysis of a mixture by spectrophotometry, without the need for extensive pre-processing of the sample. An example is the determination of bilirubin in blood plasma samples. The spectrum of pure bilirubin is known, so the molar absorbance is known. Measurements are made at one wavelength that is nearly unique for bilirubin and at a second wavelength in order to correct for possible interferences.The concentration is given by c = Acorrected / ε.
For a more complicated example, consider a mixture in solution containing two components at concentrations c1 and c2. The absorbance at any wavelength, λ is, for unit path length, given by
- <math>A(\lambda)=c_1\ \epsilon_1(\lambda)+c_2\ \epsilon_2(\lambda)</math>
Therefore, measurements at two wavelengths yields two equations in two unknowns and will suffice to determine the concentrations c1 and c2 as long as the molar aborbances of the two components, ε1 and ε1 are known at both wavelengths. In practice it is better to use linear least squares to determine the two concentrations from measurements made at more than two wavelengths. Mixtures containing more than two components can be analysed in the same way, using a minimum of n wavelengths for a mixture containing n components.
Beer–Lambert law in the atmosphere
This law is also applied to describe the attenuation of solar or stellar radiation as it travels through the atmosphere. In this case, there is scattering of radiation as well as absorption. The Beer–Lambert law for the atmosphere is usually written
- <math>I = I_0\,\exp(-m(\tau_a+\tau_g+\tau_{\rm NO_2}+\tau_w+\tau_{\rm O_3}+\tau_r)),</math>
where each <math>\tau_{x}</math> is the optical depth whose subscript identifies the source of the absorption or scattering it describes:
- <math>a</math> refers to aerosols (that absorb and scatter)
- <math>g</math> are uniformly mixed gases (mainly carbon dioxide (<math>\mathrm{CO}_2</math>) and molecular oxygen (<math>\mathrm{O}_2</math>) which only absorb)
- <math>\mathrm{NO}_2</math> is nitrogen dioxide, mainly due to urban pollution (absorption only)
- <math>w</math> is water vapour absorption
- <math>\mathrm{O}_3</math> is ozone (absorption only)
- <math>r</math> is Rayleigh scattering from molecular oxygen (<math>\mathrm{O}_2</math>) and nitrogen (<math>\mathrm{N}_2</math>) (responsible for the blue color of the sky).
<math>m</math> is the optical mass or airmass factor, a term approximately equal (for small and moderate values of <math>\theta</math>) to <math>1/\cos(\theta)</math>, where <math>\theta</math> is the observed object's zenith angle (the angle measured from the direction perpendicular to the Earth's surface at the observation site).
This equation can be used to retrieve <math>\tau_{a}</math>, the aerosol optical thickness, which is necessary for the correction of satellite images and also important in accounting for the role of aerosols in climate.
History
The law was discovered by Pierre Bouguer before 1729. It is often mis-attributed to Johann Heinrich Lambert, who cited Bouguer's “Essai d'Optique sur la Gradation de la Lumiere” (Claude Jombert, Paris, 1729) — and even quoted from it — in his “Photometria” in 1760. Much later, August Beer extended the exponential absorption law in 1852 to include the concentration of solutions in the absorption coefficient.
External links
References
- ↑ J. D. J. Ingle and S. R. Crouch, Spectrochemical Analysis, Prentice Hall, New Jersey (1988)
See also
- Atomic absorption spectroscopy
- Absorption spectroscopy
- Cavity Ring Down Spectroscopy (CRDS)
- Laser absorption spectrometry
- Logarithm
- Scientific laws named after people
- Quantification of nucleic acids
- Tunable Diode Laser Absorption Spectroscopy (TDLAS)
ar:قانون بوغير de:Lambert-Beersches Gesetz it:Legge di Lambert-Beer nl:Wet van Lambert-Beer sk:Lambertov-Beerov zákon sl:Absorpcijski zakon fi:Beerin ja Lambertin laki sv:Beer-Lamberts lag Template:WH Template:WS