Bacitracin (opthalmic): Difference between revisions
Adeel Jamil (talk | contribs) (Created page with "{{DrugProjectFormSinglePage |authorTag={{AJ}} |indicationType=treatment |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<i><span st...") |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (5 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{AJ}} | |authorTag={{AJ}} | ||
|genericName=Bacitracin | |||
|aOrAn=a | |||
|drugClass=antibiotic | |||
|indicationType=treatment | |indicationType=treatment | ||
|indication=superficial ocular infections involving the [[conjunctiva]] and/or [[cornea]] caused by Bacitracin susceptible organisms. | |||
|adverseReactions=contact dermatitis | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=* For the treatment of superficial ocular infections involving the [[conjunctiva]] and/or [[cornea]] caused by Bacitracin susceptible organisms. | |||
====Dosing Information==== | |||
* The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bacitracin (opthalmic) in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bacitracin (opthalmic) in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bacitracin (opthalmic) in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bacitracin (opthalmic) in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bacitracin (opthalmic) in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Bacitracin (opthalmic) in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bacitracin (opthalmic) in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Bacitracin (opthalmic) in pediatric patients. | ||
|contraindications=* This product should not be used in patients with a history of hypersensitivity to Bacitracin. | |||
|warnings=====PRECAUTIONS:==== | |||
* Bacitracin ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted. | |||
|clinicalTrials=* Contact dermatitis | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 457285800 | |||
| IUPAC_name = (4''R'')-4-[(2''S'')-2-({2-[(1''S'')-1-amino-2-methylbutyl]- 4,5-dihydro-1,3-thiazol-5-yl}formamido)-4-methylpentanamido]-4-{[(1''S'')- 1-{[(3''S'',6''R'',9''S'',12''R'',15''S'',18''R'',21''S'')- 18-(3-aminopropyl)-12-benzyl-15-(butan-2-yl)-3-(carbamoylmethyl)- 6-(carboxymethyl)-9-(1''H''-imidazol-5-ylmethyl)-2,5,8,11,14,17,20- heptaoxo-1,4,7,10,13,16,19-heptaazacyclopentacosan-21-yl]carbamoyl}- 2-methylbutyl]carbamoyl}butanoic acid | |||
| image = Bacitracin A.png | |||
| image2 = Bacitracin ball-and-stick.png | |||
<!--Clinical data--> | |||
| tradename = Baciim | |||
| Drugs.com = {{drugs.com|monograph|bacitracin}} | |||
| pregnancy_AU = D | |||
| pregnancy_US = C | |||
| legal_AU = S4 | |||
| legal_US = Rx-only | |||
| routes_of_administration = [[Topical]], [[Intramuscular injection|intramuscular]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 1405-87-4 | |||
| ATC_prefix = D06 | |||
| ATC_suffix = AX05 | |||
| ATC_supplemental = {{ATC|J01|XX10}} {{ATC|R02|AB04}} {{ATCvet|A07|AA93}} | |||
| PubChem = 439542 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00626 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 10481985 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 58H6RWO52I | |||
| KEGG_Ref = {{keggcite|changed|kegg}} | |||
| KEGG = D00128 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1200558 | |||
<!--Chemical data--> | |||
| C=66 | H=103 | N=17 | O=16 | S=1 | |||
| molecular_weight = 1422.69 g/mol | |||
| smiles = O=C(O)C[C@H]3NC(=O)[C@H](Cc1cncn1)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCN)NC(=O)[C@H](CCCCNC(=O)[C@H](CC(N)=O)NC3=O)NC(=O)[C@@H](NC(=O)[C@@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)C4N=C(SC4)\nC(N)C(C)CC)C(C)CC)C(C)CC | |||
| InChI = 1/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36?,37?,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1 | |||
| InChIKey = CLKOFPXJLQSYAH-NVOBBBONBV | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36?,37?,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = CLKOFPXJLQSYAH-NVOBBBONSA-N | |||
}} | |||
|mechAction=* The antibiotic, Bacitracin, exerts a profound action against many gram-positive pathogens, including the common Streptococci and Staphylococci. It is also destructive for certain gram-negative organisms. It is ineffective against fungi. | |||
|howSupplied=NDC 48102-007-13 3 - 1 g sterile tamper evident tubes with ophthalmic tip. | |||
NDC 48102-007-35 3.5 g (1/8 oz.) sterile tamper evident tubes with ophthalmic tip. | |||
|storage=* Store at 20°-25° C (68°-77° F) (See USP Controlled room temperature). | |||
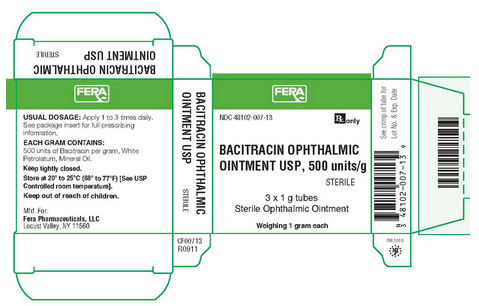
|packLabel======PRINCIPAL DISPLAY PANEL - 3X1 G TUBES, CARTON LABEL===== | |||
NDC 48102-007-13 | |||
Rx only | |||
BACITRACIN OPHTHALMIC | |||
OINTMENT USP, 500 units/g | |||
STERILE | |||
3 x 1 g tubes | |||
Sterile Ophthalmic Ointment | |||
Weighing 1 gram each | |||
USUAL DOSAGE: Apply 1 to 3 times daily. | |||
See package insert for full prescribing information. | |||
EACH GRAM CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil. | |||
Keep tightly closed. | |||
Store at 20°-25° C (68°-77° F) (See USP | |||
Controlled room temperature). | |||
Keep out of reach of children. | |||
[[File:Bacitracin opth drug label01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
=====PRINCIPAL DISPLAY PANEL - 1 G TUBE, IMMEDIATE CONTAINER LABEL===== | |||
NDC 48102-007-11 | |||
STERILE | |||
Rx only | |||
BACITRACIN | |||
OPHTHALMIC | |||
OINTMENT USP | |||
500 units/g | |||
NET WT 1 gm | |||
[[File:Bacitracin opth drug label02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
=====PRINCIPAL DISPLAY PANEL - 3.5 G TUBE, CARTON LABEL===== | |||
NDC 48102-007-35 | |||
Rx only | |||
BACITRACIN OPHTHALMIC | |||
OINTMENT USP | |||
STERILE | |||
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil. | |||
NET WT 3.5 g (1/8 Oz) | |||
USUAL DOSAGE: 3 applications daily. | |||
See insert for complete information. | |||
KEEP TIGHTLY CLOSED | |||
STORE AT ROOM TEMPERATURE | |||
KEEP OUT OF REACH OF CHILDREN | |||
See crimp of tube for Lot No. & Exp. Date | |||
[[File:Bacitracin opth drug label03.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
=====PRINCIPAL DISPLAY PANEL - 3.5 G TUBE, IMMEDIATE CONTAINER LABEL===== | |||
NDC 48102-007-35 | |||
BACITRACIN | |||
OPHTHALMIC | |||
OINTMENT USP | |||
STERILE | |||
Rx only | |||
USUAL DOSAGE: 3 applications daily. | |||
See insert for complete information. | |||
WARNING: Keep out of reach of children. | |||
See crimp for Lot No. and Exp. Date | |||
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil. | |||
NET WT 3.5 g (1/8 Oz) | |||
KEEP TIGHTLY CLOSED | |||
STORE AT ROOM TEMPERATURE | |||
[[File:Bacitracin opth drug label04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|alcohol=Alcohol-Bacitracin (opthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Bacitracin (opthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Ocu-Tracin | |||
}} | }} | ||
Latest revision as of 06:22, 18 May 2015
{{DrugProjectFormSinglePage |authorTag=Adeel Jamil, M.D. [1] |genericName=Bacitracin |aOrAn=a |drugClass=antibiotic |indicationType=treatment |indication=superficial ocular infections involving the conjunctiva and/or cornea caused by Bacitracin susceptible organisms. |adverseReactions=contact dermatitis |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=* For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by Bacitracin susceptible organisms.
Dosing Information
- The ointment should be applied directly into the conjunctival sac 1 to 3 times daily. In blepharitis all scales and crusts should be carefully removed and the ointment then spread uniformly over the lid margins. Patients should be instructed to take appropriate measures to avoid gross contamination of the ointment when applying the ointment directly to the infected eye.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Bacitracin (opthalmic) in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Bacitracin (opthalmic) in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Bacitracin (opthalmic) in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Bacitracin (opthalmic) in pediatric patients. |contraindications=* This product should not be used in patients with a history of hypersensitivity to Bacitracin. |warnings=====PRECAUTIONS:====
- Bacitracin ophthalmic ointment should not be used in deep-seated ocular infections or in those that are likely to become systemic. The prolonged use of antibiotic containing preparations may result in overgrowth of nonsusceptible organisms particularly fungi. If new infections develop during treatment appropriate antibiotic or chemotherapy should be instituted.
|clinicalTrials=* Contact dermatitis |drugBox={{Drugbox2 | Verifiedfields = changed | verifiedrevid = 457285800 | IUPAC_name = (4R)-4-[(2S)-2-({2-[(1S)-1-amino-2-methylbutyl]- 4,5-dihydro-1,3-thiazol-5-yl}formamido)-4-methylpentanamido]-4-{[(1S)- 1-{[(3S,6R,9S,12R,15S,18R,21S)- 18-(3-aminopropyl)-12-benzyl-15-(butan-2-yl)-3-(carbamoylmethyl)- 6-(carboxymethyl)-9-(1H-imidazol-5-ylmethyl)-2,5,8,11,14,17,20- heptaoxo-1,4,7,10,13,16,19-heptaazacyclopentacosan-21-yl]carbamoyl}- 2-methylbutyl]carbamoyl}butanoic acid | image = Bacitracin A.png | image2 = Bacitracin ball-and-stick.png
| tradename = Baciim | Drugs.com = Monograph | pregnancy_AU = D | pregnancy_US = C | legal_AU = S4 | legal_US = Rx-only | routes_of_administration = Topical, intramuscular
| bioavailability = | protein_bound = | metabolism = | elimination_half-life =
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 1405-87-4
| ATC_prefix = D06
| ATC_suffix = AX05
| ATC_supplemental = J01XX10 (WHO) R02AB04 (WHO) Template:ATCvet
| PubChem = 439542
| DrugBank_Ref =
| DrugBank = DB00626
| ChemSpiderID_Ref =
| ChemSpiderID = 10481985
| UNII_Ref =
| UNII = 58H6RWO52I
| KEGG_Ref =
| KEGG = D00128
| ChEMBL_Ref =
| ChEMBL = 1200558
| C=66 | H=103 | N=17 | O=16 | S=1
| molecular_weight = 1422.69 g/mol
| smiles = O=C(O)C[C@H]3NC(=O)[C@H](Cc1cncn1)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCN)NC(=O)[C@H](CCCCNC(=O)[C@H](CC(N)=O)NC3=O)NC(=O)[C@@H](NC(=O)[C@@H](CCC(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)C4N=C(SC4)\nC(N)C(C)CC)C(C)CC)C(C)CC
| InChI = 1/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36?,37?,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1
| InChIKey = CLKOFPXJLQSYAH-NVOBBBONBV
| StdInChI_Ref =
| StdInChI = 1S/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36?,37?,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1
| StdInChIKey_Ref =
| StdInChIKey = CLKOFPXJLQSYAH-NVOBBBONSA-N
}}
|mechAction=* The antibiotic, Bacitracin, exerts a profound action against many gram-positive pathogens, including the common Streptococci and Staphylococci. It is also destructive for certain gram-negative organisms. It is ineffective against fungi.
|howSupplied=NDC 48102-007-13 3 - 1 g sterile tamper evident tubes with ophthalmic tip.
NDC 48102-007-35 3.5 g (1/8 oz.) sterile tamper evident tubes with ophthalmic tip. |storage=* Store at 20°-25° C (68°-77° F) (See USP Controlled room temperature). |packLabel======PRINCIPAL DISPLAY PANEL - 3X1 G TUBES, CARTON LABEL=====
NDC 48102-007-13
Rx only
BACITRACIN OPHTHALMIC OINTMENT USP, 500 units/g STERILE
3 x 1 g tubes
Sterile Ophthalmic Ointment
Weighing 1 gram each
USUAL DOSAGE: Apply 1 to 3 times daily. See package insert for full prescribing information.
EACH GRAM CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
Keep tightly closed.
Store at 20°-25° C (68°-77° F) (See USP
Controlled room temperature).
Keep out of reach of children.
PRINCIPAL DISPLAY PANEL - 1 G TUBE, IMMEDIATE CONTAINER LABEL
NDC 48102-007-11
STERILE
Rx only
BACITRACIN
OPHTHALMIC OINTMENT USP
500 units/g
NET WT 1 gm

PRINCIPAL DISPLAY PANEL - 3.5 G TUBE, CARTON LABEL
NDC 48102-007-35
Rx only
BACITRACIN OPHTHALMIC OINTMENT USP STERILE
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
NET WT 3.5 g (1/8 Oz)
USUAL DOSAGE: 3 applications daily. See insert for complete information.
KEEP TIGHTLY CLOSED STORE AT ROOM TEMPERATURE KEEP OUT OF REACH OF CHILDREN
See crimp of tube for Lot No. & Exp. Date

PRINCIPAL DISPLAY PANEL - 3.5 G TUBE, IMMEDIATE CONTAINER LABEL
NDC 48102-007-35
BACITRACIN OPHTHALMIC OINTMENT USP STERILE
Rx only
USUAL DOSAGE: 3 applications daily. See insert for complete information.
WARNING: Keep out of reach of children.
See crimp for Lot No. and Exp. Date
CONTAINS: 500 units of Bacitracin per gram, White Petrolatum, Mineral Oil.
NET WT 3.5 g (1/8 Oz)
KEEP TIGHTLY CLOSED STORE AT ROOM TEMPERATURE

|alcohol=Alcohol-Bacitracin (opthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=* Ocu-Tracin }}