Bacillus anthracis
| style="background:#Template:Taxobox colour;"|Bacillus anthracis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
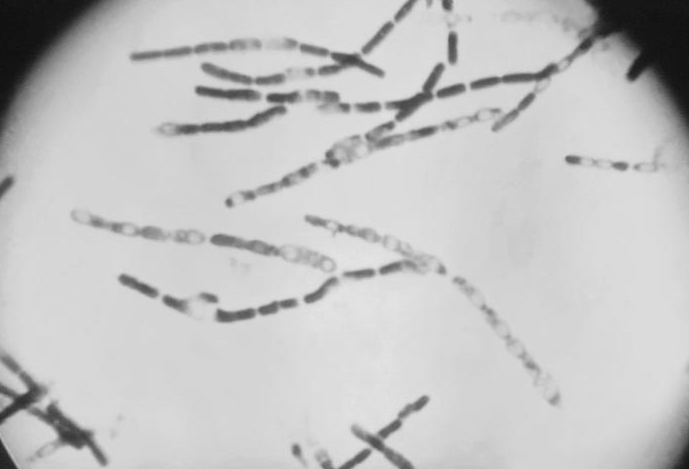
| Photomicrograph of Bacillus anthracis (fuchsin-methylene blue spore stain) Photomicrograph of Bacillus anthracis (fuchsin-methylene blue spore stain)
| ||||||||||||||
| style="background:#Template:Taxobox colour;" | Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Bacillus anthracis Cohn 1872 |
|
Anthrax Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Bacillus anthracis On the Web |
|
American Roentgen Ray Society Images of Bacillus anthracis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The causative agent of anthrax is B. anthracis, a nonmotile, Gram-positive, aerobic or facultatively anaerobic, endospore-forming, rod-shaped bacterium. The spores of B. anthracis, which can remain dormant in the environment for decades, are the infectious form, but this vegetative B. anthracis rarely causes disease.[1] The Bacillus may enter the body through the skin, lungs, gastrointestinal system or by injection, after which they will travel to the lymph nodes. The virulence factors will facilitate the translocation of the toxins to the cytosol. The natural reservoirs of Bacillus anthracis include humans, mammals, herbivores, reptiles, and birds.
Historical background
French physician Casimir Davaine (1812-1882) demonstrated the symptoms of anthrax were invariably accompanied by the microbe B. anthracis.[2] German physician Aloys Pollender (1799–1879) is also credited for this discovery. B. anthracis was the first bacterium conclusively demonstrated to cause disease, by Robert Koch in 1876.[3] The species name anthracis is from the Greek anthrax (ἄνθραξ), meaning "coal" and referring to the most common form of the disease, cutaneous anthrax, in which large, black skin lesions are formed.
Biology
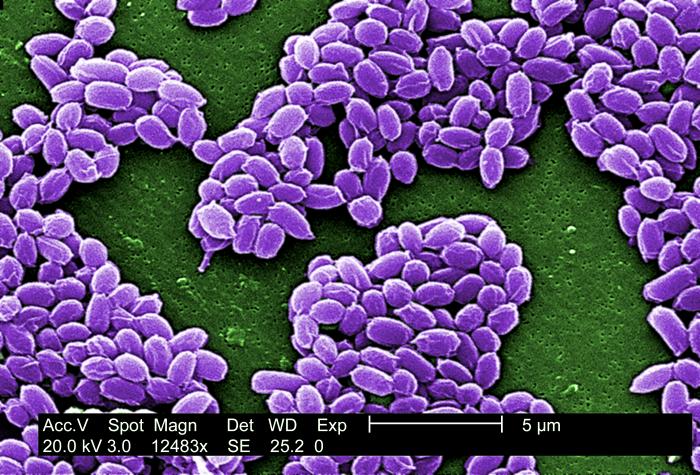
B. anthracis, the causative agent of anthrax, is a nonmotile, Gram-positive, aerobic or facultatively anaerobic, endospore-forming, rod-shaped bacterium approximately 4 μm by 1 μm, although under the microscope it frequently appears in chains of cells. Like other Bacillus, Bacillus anthracis is saprophyte, being able to live in vegetation, air, water and soil.[4]
These bacterial cells may occur isolated, form groups of 2 or more cells in the body, or long chains in cultures.[4] In blood smears, smears of tissues or lesion fluid from diagnostic specimens, these chains are two to a few cells in length. In smears made from in vitro cultures, they can appear as endless strings of cells - responsible for the characteristic tackiness of the colonies and for the flocculating nature of broth cultures. Cell cultures appear with a large, grey and curled structure, resembling a "medusa head".[4]
B. anthracis have a characteristic square-ended appearance, traditionally associated with its vegetative state, although this may not always be very clear. In the presence of oxygen, ideally at 32 - 35 ºC, and towards the end of the exponential phase of growth, one ellipsoidal spore (approximately 2 μm by 1 μm in size) is formed within each cell.[5][4] Commonly the spores will be produced once the cell senses scarcity of nutrients.[6]
The spores of B. anthracis, which can remain dormant in the environment for decades, being resistant to heat and disinfectants, are the infectious form, but vegetative B. anthracis rarely causes disease.[7][4]
In the absence of oxygen and under a high partial pressure of Co2, in the presence of bicarbonate, the vegetative cell secretes its polypeptide capsule. This is one of the two established in vivo virulence factors of B. anthracis. The capsule is also a primary diagnostic aid.[5] Protective antigen (PA) and edema factor (EF) combine to form edema toxin (ET) and PA and lethal factor (LF) combine to form lethal toxin (LT), the active toxins.[8][9]
 |
 |
Genome structure
B. anthracis has a single chromosome which is a circular, 5,227,293-bp DNA molecule.[12] It also has two circular, extrachromosomal, double-stranded DNA plasmids, pXO1 and pXO2. Both the pXO1 and pXO2 plasmids are required for full virulence and represent two distinct plasmid families.[13]
| Feature | Chromosome | pXO1 | pXO2 |
|---|---|---|---|
| Size (bp) | 5,227,293 | 181,677 | 94,829 |
| Number of genes | 5,508 | 217 | 113 |
| Replicon coding (%) | 84.3 | 77.1 | 76.2 |
| Average gene length (nt) | 800 | 645 | 639 |
| G+C content (%) | 35.4 | 32.5 | 33.0 |
| rRNA operons | 11 | 0 | 0 |
| tRNAs | 95 | 0 | 0 |
| sRNAs | 3 | 2 | 0 |
| Phage genes | 62 | 0 | 0 |
| Transposon genes | 18 | 15 | 6 |
| Disrupted reading frame | 37 | 5 | 7 |
| Genes with assigned function | 2,762 | 65 | 38 |
| Conserved hypothetical genes | 1,212 | 22 | 19 |
| Genes of unknown function | 657 | 8 | 5 |
| Hypothetical genes | 877 | 122 | 51 |
pXO1 plasmid
The pXO1 plasmid (182 kb) contains the genes that encode for the anthrax toxin components: pag (protective antigen, PA), lef (lethal factor, LF), and cya (edema factor, EF). These factors are contained within a 44.8-kb pathogenicity island (PAI). The lethal toxin is a combination of PA with LF and the edema toxin is a combination of PA with EF. The PAI also contains genes which encode a transcriptional activator AtxA and the repressor PagR, both of which regulate the expression of the anthrax toxin genes.[13]
pXO2 plasmid
pXO2 encodes a five-gene operon (capBCADE) which synthesizes a poly-γ-D-glutamic acid (polyglutamate) capsule. This capsule allows B. anthracis to evade the host immune system by protecting itself from phagocytosis. Expression of the capsule operon is activated by the transcriptional regulators AcpA and AcpB, located in the pXO2 pathogenicity island (35 kb). Interestingly, AcpA and AcpB expression are under the control of AtxA from pXO1.[13]
Strains
The 89 known strains of B. anthracis include:
- Sterne strain (34F2; aka the "Weybridge strain"), used by Max Sterne in his 1930s vaccines
- Vollum strain, formerly weaponized by the US, UK, and Iraq; isolated from cow in Oxfordshire, UK, in 1935
- Vollum M-36, virulent British research strain; passed through macaques 36 times
- Vollum 1B, weaponized by the US and UK in the 1940s-60s
- Vollum-14578, UK biotesting contaminated Gruinard Island, Scotland, in 1940s
- V770-NP1-R, the avirulent, nonencapsulated strain used in the BioThrax vaccine
- Anthrax 836, highly virulent strain weaponized by the USSR; discovered in Kirov in 1953
- Ames strain, isolated from a cow in Texas in 1981; famously used in AMERITHRAX letter attacks (2001)
- Ames Ancestor
- Ames Florida
- H9401, isolated from human patient in Korea; used in investigational anthrax vaccines[14]
Evolution
Whole genome sequencing has made reconstruction of the B. anthracis phylogeny extremely accurate. A contributing factor to the reconstruction is B. anthracis being monomorphic, meaning it has low genetic diversity, including the absence of any measurable lateral DNA transfer since its derivation as a species. The lack of diversity is due to a short evolutionary history that has precluded mutational saturation in single nucleotide polymorphisms.[15]
A short evolutionary time does not necessarily mean a short chronological time. When DNA is replicated, mistakes occur which become genetic mutations. The buildup of these mutations over time leads to the evolution of a species. During the B. anthracis lifecycle, it spends a significant amount of time in the soil spore reservoir stage, a stage in which DNA replication does not occur. These prolonged periods of dormancy have greatly reduced the evolutionary rate of the organism.[15]
Nearest neighbors
B. anthracis belongs to the B. cereus group consisting of the strains: B. cereus, B. anthracis, B. thuringiensis, B. weihenstephanensis, B. mycoides, and B. pseudomycoides. The first three strains are pathogenic or opportunistic to insects or mammals, while the last three are not considered pathogenic. The strains of this group are genetically and phenotypically heterogeneous overall, but some of the strains are more closely related and phylogenetically intermixed at the chromosome level. The B. cereus group generally exhibits complex genomes and most carry varying numbers of plasmids.[13]
B. cereus is a soil-dwelling bacterium which can colonize the gut of invertebrates as a symbiont[16] and is a frequent cause of food poisoning[17] It produces an emetic toxin, enterotoxins, and other virulence factors.[18] The enterotoxins and virulence factors are encoded on the chromosome, while the emetic toxin is encoded on a 270-kb plasmid, pCER270.[13]
B. thuringiensis is an insect pathogen and is characterized by production of parasporal crystals of insecticidal toxins Cry and Cyt.[19] The genes encoding these proteins are commonly located on plasmids which can be lost from the organism, making it indistinguishable from B. cereus.[13]
Pseudogene
PlcR is a global transcriptional regulator which controls most of the secreted virulence factors in B. cereus and B. thuringiensis. It is chromosomally encoded and is ubiquitous throughout the cell.[20] In B. anthracis, however, the plcR gene contains a single base change at position 640, a nonsense mutation, which creates a dysfunctional protein. While 1% of the B. cereus group carries an inactivated plcR gene, none of them carries the specific mutation found only in B. anthracis.[21]
The plcR gene is part of a two-gene operon with papR.[22][23] The papR gene encodes a small protein which is secreted from the cell and the reimported as a processed heptapeptide forming a quorum-sensing system.[23][24] The lack of PlcR in B. anthracis is a principle characteristic differentiating it from other members of the B. cereus group. While B. cereus and B. thuringiensis depend on the plcR gene for expression of their virulence factors, B. anthracis relies on the pXO1 and pXO2 plasmids for its virulence.[13]
Laboratory research
Components of tea, such as polyphenols, have the ability to inhibit the activity both of B. anthracis and its toxin considerably; spores, however, are not affected. The addition of milk to the tea completely inhibits its antibacterial activity against anthrax.[25] Activity against the B. athracis in the laboratory does not prove that drinking tea affects the course of an infection, since it is unknown how these polyphenols are absorbed and distributed within the body.
Recent research
Advances in genotyping methods have led to improved genetic analysis for variation and relatedness. These methods include multiple-locus variable-number tandem repeat analysis (MLVA) and typing systems using canonical single-nucleotide polymorphisms. The Ames ancestor chromosome was sequenced in 2003[12] and contributes to the identification of genes involved in the virulence of B. anthracis. Recently, B. anthracis isolate H9401 was isolated from a Korean patient suffering from gastrointestinal anthrax. The goal of the Republic of Korea is to use this strain as a challenge strain to develop a recombinant vaccine against anthrax.[14]
The H9401 strain isolated in the Republic of Korea was sequenced using 454 GS-FLX technology and analyzed using several bioinformatics tools to align, annotate, and compare H9401 to other B. anthracis strains. The sequencing coverage level suggests a molecular ratio of pXO1:pXO2:chromosome as 3:2:1 which is identical to the Ames Florida and Ames Ancestor strains. H9401 has 99.679% sequence homology with Ames Ancestor with an amino acid sequence homology of 99.870%. H9401 has a circular chromosome (5,218,947 bp with 5,480 predicted ORFs), the pXO1 plasmid (181,700 bp with 202 predicted ORFs), and the pXO2 plasmid (94,824 bp with 110 predicted ORFs).[14] As compared to the Ames Ancestor chromosome above, the H9401 chromosome is about 8.5 kb smaller. Due to the high pathogenecity and sequence similarity to the Ames Ancestor, H9401 will be used as a reference for testing the efficacy of candidate anthrax vaccines by the Repbulic of Korea.[14]
Host interactions
As with most other pathogenic bacteria, B. anthracis must acquire iron to grow and proliferate in its host environment. The most readily available iron sources for pathogenic bacteria are the heme groups used by the host in the transport of oxygen. To scavenge heme from host hemoglobin and myoglobin, B. anthracis uses two secretory siderophore proteins, IsdX1 and IsdX2. These proteins can separate heme from hemoglobin, allowing surface proteins of B. anthracis to transport it into the cell.[26]
Origin
Bacillus anthracis is thought to have originated in Egypt and Mesopotamia. Many scholars think that in Moses’ time, during the 10 plagues of Egypt, anthrax may have caused what was known as the fifth plague, described as a sickness affecting horses, cattle, sheep, camels and oxen.
Tropism
After entering the body (through the skin, lungs, gastrointestinal tract or by injection), B. anthracis spores are believed to germinate locally or be transported by phagocytic cells to the lymphatics and regional lymph nodes, where they germinate.[9][27] After binding to cell surface receptors, the PA portion of the complexes facilitates translocation of the toxins to the cytosol.[28][9]
Natural Reservoir
Natural reservoirs of Bacillus anthracis include:[5][4][9]
- Humans
- Mammals
- Herbivores
- Reptiles
- Birds
References
- ↑ Sean V. Shadomy & Theresa L. Smith (2008). "Zoonosis update. Anthrax". Journal of the American Veterinary Medical Association. 233 (1): 63–72. doi:10.2460/javma.233.1.63. PMID 18593313. Unknown parameter
|month=ignored (help) - ↑ Théodoridès, J (April 1966). "Casimir Davaine (1812-1882): a precursor of Pasteur". Medical history. 10 (2): 155–65. doi:10.1017/S0025727300010942. PMC 1033586. PMID 5325873.
- ↑ Koch, R. (1876) "Untersuchungen über Bakterien: V. Die Ätiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte des Bacillus anthracis" (Investigations into bacteria: V. The etiology of anthrax, based on the ontogenesis of Bacillus anthracis), Cohns Beitrage zur Biologie der Pflanzen, vol. 2, no. 2, pages 277–310.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Bhatnagar, Rakesh; Batra, Smriti (2001). "Anthrax Toxin". Critical Reviews in Microbiology. 27 (3): 167–200. doi:10.1080/20014091096738. ISSN 1040-841X.
- ↑ 5.0 5.1 5.2 "Anthrax in Humans and Animals" (PDF).
- ↑ Spencer RC (2003). "Bacillus anthracis". J Clin Pathol. 56 (3): 182–7. PMC 1769905. PMID 12610093.
- ↑ Sean V. Shadomy & Theresa L. Smith (2008). "Zoonosis update. Anthrax". Journal of the American Veterinary Medical Association. 233 (1): 63–72. doi:10.2460/javma.233.1.63. PMID 18593313. Unknown parameter
|month=ignored (help) - ↑ Mahtab Moayeri & Stephen H. Leppla (2004). "The roles of anthrax toxin in pathogenesis". Current opinion in microbiology. 7 (1): 19–24. doi:10.1016/j.mib.2003.12.001. PMID 15036135. Unknown parameter
|month=ignored (help) - ↑ 9.0 9.1 9.2 9.3 "Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults".
- ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ 12.0 12.1 Read, TD (May 1, 2003). "The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria". Nature. 423 (6935): 81–6. doi:10.1038/nature01586. PMID 12721629. Unknown parameter
|coauthors=ignored (help) - ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 Kolstø, Anne-Brit; Tourasse, Nicolas J.; Økstad, Ole Andreas (1 October 2009). "What Sets Apart from Other Species?". Annual Review of Microbiology. 63 (1): 451–476. doi:10.1146/annurev.micro.091208.073255.
- ↑ 14.0 14.1 14.2 14.3 Chun, J.-H.; Hong, K.-J.; Cha, S. H.; Cho, M.-H.; Lee, K. J.; Jeong, D. H.; Yoo, C.-K.; Rhie, G.-e. (18 July 2012). "Complete Genome Sequence of Bacillus anthracis H9401, an Isolate from a Korean Patient with Anthrax". Journal of Bacteriology. 194 (15): 4116–4117. doi:10.1128/JB.00159-12. PMC 3416559. PMID 22815438.
- ↑ 15.0 15.1 Keim, Paul; Gruendike, Jeffrey M.; Klevytska, Alexandra M.; Schupp, James M.; Challacombe, Jean; Okinaka, Richard (1 December 2009). "The genome and variation of Bacillus anthracis". Molecular Aspects of Medicine. 30 (6): 397–405. doi:10.1016/j.mam.2009.08.005. PMC 3034159. PMID 19729033.
- ↑ Jensen, G. B.; Hansen, B. M.; Eilenberg, J.; Mahillon, J. (18 July 2003). "The hidden lifestyles of Bacillus cereus and relatives". Environmental Microbiology. 5 (8): 631–640. doi:10.1046/j.1462-2920.2003.00461.x. PMID 12871230.
- ↑ Drobniewski, FA (October 1993). "Bacillus cereus and related species". Clinical Microbiology Reviews. 6 (4): 324–38. PMC 358292. PMID 8269390.
- ↑ Stenfors Arnesen, Lotte P.; Fagerlund, Annette; Granum, Per Einar (1 July 2008). "From soil to gut: and its food poisoning toxins". FEMS Microbiology Reviews. 32 (4): 579–606. doi:10.1111/j.1574-6976.2008.00112.x. PMID 18422617.
- ↑ Schnepf, E; Crickmore, N; Van Rie, J; Lereclus, D; Baum, J; Feitelson, J; Zeigler, DR; Dean, DH (September 1998). "Bacillus thuringiensis and its pesticidal crystal proteins". Microbiology and molecular biology reviews : MMBR. 62 (3): 775–806. PMC 98934. PMID 9729609.
- ↑ Agaisse, H; Gominet, M; Okstad, OA; Kolstø, AB; Lereclus, D (June 1999). "PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis". Molecular microbiology. 32 (5): 1043–53. doi:10.1046/j.1365-2958.1999.01419.x. PMID 10361306.
- ↑ Slamti, L; Perchat, S; Gominet, M; Vilas-Bôas, G; Fouet, A; Mock, M; Sanchis, V; Chaufaux, J; Gohar, M; Lereclus, D (June 2004). "Distinct mutations in PlcR explain why some strains of the Bacillus cereus group are nonhemolytic". Journal of bacteriology. 186 (11): 3531–8. doi:10.1128/JB.186.11.3531-3538.2004. PMC 415780. PMID 15150241.
- ↑ Okstad, OA; Gominet, M; Purnelle, B; Rose, M; Lereclus, D; Kolstø, AB (November 1999). "Sequence analysis of three Bacillus cereus loci carrying PIcR-regulated genes encoding degradative enzymes and enterotoxin". Microbiology (Reading, England). 145 (11): 3129–38. PMID 10589720.
- ↑ 23.0 23.1 Slamti, L; Lereclus, D (Sep 2, 2002). "A cell-cell signaling peptide activates the PlcR virulence regulon in bacteria of the Bacillus cereus group". The EMBO Journal. 21 (17): 4550–9. doi:10.1093/emboj/cdf450. PMC 126190. PMID 12198157.
- ↑ Bouillaut, L; Perchat, S; Arold, S; Zorrilla, S; Slamti, L; Henry, C; Gohar, M; Declerck, N; Lereclus, D (June 2008). "Molecular basis for group-specific activation of the virulence regulator PlcR by PapR heptapeptides". Nucleic Acids Research. 36 (11): 3791–801. doi:10.1093/nar/gkn149. PMC 2441798. PMID 18492723.
- ↑ "Anthrax and tea". Society for Applied Microbiology. 2011-12-21. Retrieved 2011-12-21.
- ↑ Maresso AW, Garufi G, Schneewind O (2008). "Bacillus anthracis Secretes Proteins That Mediate Heme Acquisition from Hemoglobin". PLOS Pathogens. 4(8): e1000132.
- ↑ Ross, Joan M. (1957). "The pathogenesis of anthrax following the administration of spores by the respiratory route". The Journal of Pathology and Bacteriology. 73 (2): 485–494. doi:10.1002/path.1700730219. ISSN 0368-3494.
- ↑ Moayeri, M (2004). "The roles of anthrax toxin in pathogenesis". Current Opinion in Microbiology. 7 (1): 19–24. doi:10.1016/j.mib.2003.12.001. ISSN 1369-5274.
External links
| Wikimedia Commons has media related to Bacillus anthracis. |
- Bacillus anthracis genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
- Hazards in Animal Research Database - Bacillus anthracis
- Pathema-Bacillus Resource
- Pages with citations using unsupported parameters
- CS1 maint: PMC format
- CS1 errors: external links
- CS1 maint: Multiple names: authors list
- Pages with script errors
- Pages with broken file links
- Commons category link is defined as the pagename
- Commons category link is on Wikidata using P373
- Anthrax
- Bacillus
- Bacteria with sequenced genomes