Azithromycin (injection)
{{DrugProjectFormSinglePage |authorTag=Ammu Susheela, M.D. [1] |genericName=Azithromycin |aOrAn=an |drugClass=antibiotic |indicationType=treatment |indication=community-acquired pneumonia, pelvic inflammatory disease |adverseReactions=abdominal pain |blackBoxWarningTitle=ConditionName: |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult=* Azithromycin for injection is indicated for the treatment of patients with infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
- Community-acquired pneumonia due to Chlamydia pneumoniae, Haemophilus influenzae, Legionella pneumophila, Moraxella catarrhalis, Mycoplasma pneumoniae, Staphylococcus aureus, or Streptococcus pneumoniae in patients who require initial intravenous therapy.
- Pelvic inflammatory disease due to Chlamydia trachomatis, Neisseria gonorrhoeae, or Mycoplasma hominis in patients who require initial intravenous therapy. If anaerobic microorganisms are suspected of contributing to the infection, an antimicrobial agent with anaerobic activity should be administered in combination with azithromycin.
- Azithromycin for injection should be followed by azithromycin by the oral route as required.
- Appropriate culture and susceptibility tests should be performed before treatment to determine the causative microorganism and its susceptibility to azithromycin. * Therapy with azithromycin may be initiated before results of these tests are known; once the results become available, antimicrobial therapy should be adjusted accordingly.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- The recommended dose of azithromycin for injection for the treatment of adult patients with community-acquired pneumonia due to the indicated organisms is: 500 mg as a single daily dose by the intravenous route for at least two days. Intravenous therapy should be followed by azithromycin by the oral route at a single, daily dose of 500 mg, administered as two 250-mg tablets to complete a 7- to 10-day course of therapy. The timing of the switch to oral therapy should be done at the discretion of the physician and in accordance with clinical response.
- The recommended dose of azithromycin for the treatment of adult patients with pelvic inflammatory disease due to the indicated organisms is: 500 mg as a single daily dose by the intravenous route for one or two days. Intravenous therapy should be followed by azithromycin by the oral route at a single, daily dose of 250 mg to complete a 7-day course of therapy. The timing of the switch to oral therapy should be done at the discretion of the physician and in accordance with clinical response. If anaerobic microorganisms are suspected of contributing to the infection, an antimicrobial agent with anaerobic activity should be administered in combination with azithromycin.
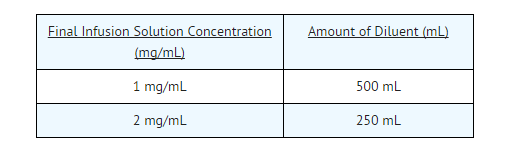
- The infusate concentration and rate of infusion for azithromycin for injection should be either 1 mg/mL over 3 hours or 2 mg/mL over 1 hour.
- Preparation of the Solution for Intravenous Administration is as Follows:
Reconstitution
- Prepare the initial solution of azithromycin for injection by adding 4.8 mL of Sterile Water For Injection to the 500 mg vial and shaking the vial until all of the drug is dissolved. Since azithromycin for injection is supplied under vacuum, it is recommended that a standard 5 mL (non-automated) syringe be used to ensure that the exact amount of 4.8 mL of Sterile Water is dispensed. Each mL of reconstituted solution contains 100 mg azithromycin. Reconstituted solution is stable for 24 hours when stored below 25°C or 77°F.
- Parenteral drug products should be inspected visually for particulate matter prior to administration. If particulate matter is evident in reconstituted fluids, the drug solution should be discarded.
- Dilute This Solution Further Prior to Administration as Instructed Below.
Dilution
- To provide azithromycin over a concentration range of 1-2 mg/mL, transfer 5 mL of the 100 mg/mL azithromycin solution into the appropriate amount of any of the diluents listed below:
- Normal Saline (0.9% sodium chloride)
- 1/2 Normal Saline (0.45% sodium chloride)
- 5% Dextrose in Water
- Lactated Ringer’s Solution
- 5% Dextrose in 1/2 Normal Saline (0.45% sodium chloride) with 20 mEq KCl
- 5% Dextrose in Lactated Ringer’s Solution
- 5% Dextrose in 1/3 Normal Saline (0.3% sodium chloride)
- 5% Dextrose in 1/2 Normal Saline (0.45% sodium chloride)
- Normosol®-M in 5% Dextrose
- Normosol®-R in 5% Dextrose
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Azithromycin (injection) in adult patients.
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Azithromycin (injection) in adult patients.
|fdaLIADPed=There is limited information regarding FDA-Labeled Use of Azithromycin (injection) in pediatric patients.
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Azithromycin (injection) in pediatric patients.
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Azithromycin (injection) in pediatric patients.
|contraindications=* Azithromycin for injection is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, or any macrolide antibiotic.
|warnings=* Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens Johnson Syndrome and toxic epidermal necrolysis have been reported rarely in patients on azithromycin therapy. Although rare, fatalities have been reported. Despite initially successful symptomatic treatment of the allergic symptoms, when symptomatic therapy was discontinued, the allergic symptoms recurred soon thereafter in some patients without further azithromycin exposure. These patients required prolonged periods of observation and symptomatic treatment. The relationship of these episodes to the long tissue half-life of azithromycin and subsequent prolonged exposure to antigen is unknown at present.
- If an allergic reaction occurs, the drug should be discontinued and appropriate therapy should be instituted. Physicians should be aware that reappearance of the allergic symptoms may occur when symptomatic therapy is discontinued.
- Pseudomembranous colitis has been reported with nearly all antibacterial agents and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated colitis."
- After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
|clinicalTrials=* In clinical trials of intravenous azithromycin for community-acquired pneumonia, in which 2-5 I.V. doses were given, most of the reported side effects were mild to moderate in severity and were reversible upon discontinuation of the drug. The majority of patients in these trials had one or more comorbid diseases and were receiving concomitant medications. Approximately 1.2% of the patients discontinued intravenous azithromycin therapy, and a total of 2.4% discontinued azithromycin therapy by either the intravenous or oral route because of clinical or laboratory side effects.
- In clinical trials conducted in patients with pelvic inflammatory disease, in which 1-2 I.V. doses were given, 2% of women who received monotherapy with azithromycin and 4% who received azithromycin plus metronidazole discontinued therapy due to clinical side effects.
- Clinical side effects leading to discontinuations from these studies were most commonly gastrointestinal (abdominal pain, nausea, vomiting, diarrhea), and rashes; laboratory side effects leading to discontinuation were increases in transaminase levels and/or alkaline phosphatase levels.
Clinical
- Overall, the most common side effects associated with treatment in adult patients who received I.V./P.O. azithromycin in studies of community-acquired pneumonia were related to the gastrointestinal system with diarrhea/loose stools (4.3%), nausea (3.9%), abdominal pain (2.7%), and vomiting (1.4%) being the most frequently reported. Approximately 12% of patients experienced a side effect related to the intravenous infusion; most common were pain at the injection site (6.5%) and local inflammation (3.1%).
- The most common side effects associated with treatment in adult women who received I.V./P.O. azithromycin in studies of pelvic inflammatory disease were related to the gastrointestinal system. Diarrhea (8.5%) and nausea (6.6%) were most commonly reported, followed by vaginitis (2.8%), abdominal pain (1.9%), anorexia (1.9%), rash and pruritus (1.9%). When azithromycin was coadministered with metronidazole in these studies, a higher proportion of women experienced side effects of nausea (10.3%), abdominal pain (3.7%), vomiting (2.8%), application site reaction, stomatitis, dizziness, or dyspnea (all at 1.9%).
- No other side effects occurred in patients on the multiple dose I.V./P.O. regimen of azithromycin in these studies with a frequency greater than 1%.
- Side effects that occurred with a frequency of 1% or less included the following:
- Gastrointestinal:
- Nervous System:
- Allergic:
- Special Senses:
- Taste perversion.
|postmarketing=* Adverse events reported with orally administered azithromycin during the post-marketing period in adult and/or pediatric patients for which a causal relationship could not be established include:
- Allergic:
- Cardiovascular:
- Arrhythmias including ventricular tachycardia, hypotension. There have been rare reports of QT prolongation and torsades de pointes.
- Gastrointestinal:
- Anorexia, constipation, dyspepsia, flatulence, vomiting/diarrhea rarely resulting in dehydration, pseudomembranous colitis, pancreatitis, oral candidiasis and rare reports of tongue discoloration.
- General:
- Asthenia, paresthesia, fatigue, malaise and anaphylaxis (rarely fatal)
- Genitourinary:
- Hematopoietic:
- Liver/Biliary:
- Abnormal liver function including hepatitis and cholestatic jaundice, as well as rare cases of hepatic necrosis and hepatic failure, some of which have resulted in death.
- Nervous System:
- Convulsions, dizziness/vertigo, headache, somnolence, hyperactivity, nervousness, agitation and syncope.
- Psychiatric:
- Aggressive reaction and anxiety.
- Skin/Appendages:
- Pruritus, rarely serious skin reactions including erythema multiforme, Stevens-Johnson Syndrome, and toxic epidermal necrolysis.
- Special Senses:
- Laboratory Abnormalities:
- Significant abnormalities (irrespective of drug relationship) occurring during the clinical trials were reported as follows:
- With an incidence of 4-6%, elevated ALT (SGPT), AST (SGOT), creatinine
- With an incidence of 1-3%, elevated LDH, bilirubin
- With an incidence of less than 1%, leukopenia, neutropenia, decreased platelet count, and elevated serum alkaline phosphatase.
- When follow-up was provided, changes in laboratory tests appeared to be reversible.
- In multiple-dose clinical trials involving more than 750 patients treated with azithromycin (I.V./P.O.), less than 2% of patients discontinued azithromycin therapy because of treatment-related liver enzyme abnormalities.
|drugInteractions=* Aluminum- and magnesium-containing antacids reduce the peak serum levels (rate) but not the AUC (extent) of orally administered azithromycin.
- Administration of cimetidine (800 mg) two hours prior to orally administered azithromycin had no effect on azithromycin absorption.
- Azithromycin given by the oral route did not affect the plasma levels or pharmacokinetics of theophylline administered as a single intravenous dose. The effect of azithromycin on the plasma levels or pharmacokinetics of theophylline administered in multiple doses resulting in therapeutic steady-state levels of theophylline is not known. However, concurrent use of macrolides and theophylline has been associated with increases in the serum concentrations of theophylline. Therefore, until further data are available, prudent medical practice dictates careful monitoring of plasma theophylline levels in patients receiving azithromycin and theophylline concomitantly.
- Azithromycin given by the oral route did not affect the prothrombin time response to a single dose of warfarin. However, prudent medical practice dictates careful monitoring of prothrombin time in all patients treated with azithromycin and warfarin concomitantly. Concurrent use of macrolides and warfarin in clinical practice has been associated with increased anticoagulant effects.
- The following drug interactions have not been reported in clinical trials with azithromycin; however, no specific drug interaction studies have been performed to evaluate potential drug-drug interaction. Nonetheless, they have been observed with macrolide products. Until further data are developed regarding drug interactions when azithromycin and these drugs are used concomitantly, careful monitoring of patients is advised:
- Digoxin - elevated digoxin levels. - Ergotamine or dihydroergotamine - acute ergot toxicity characterized by severe peripheral vasospasm and dysesthesia. - Triazolam - Increased pharmacologic effect of triazolam by decreasing the clearance of triazolam. - Drugs metabolized by the cytochrome P450 system – elevations of serum carbamazepine, terfenadine, cyclosporine, hexobarbital, and phenytoin levels. Aluminum- and magnesium-containing antacids reduce the peak serum levels (rate) but not the AUC (extent) of orally administered azithromycin.
- Administration of cimetidine (800 mg) two hours prior to orally administered azithromycin had no effect on azithromycin absorption.
- Azithromycin given by the oral route did not affect the plasma levels or pharmacokinetics of theophylline administered as a single intravenous dose. The effect of azithromycin on the plasma levels or pharmacokinetics of theophylline administered in multiple doses resulting in therapeutic steady-state levels of theophylline is not known. However, concurrent use of macrolides and theophylline has been associated with increases in the serum concentrations of theophylline. Therefore, until further data are available, prudent medical practice dictates careful monitoring of plasma theophylline levels in patients receiving azithromycin and theophylline concomitantly.
- Azithromycin given by the oral route did not affect the prothrombin time response to a single dose of warfarin. However, prudent medical practice dictates careful monitoring of prothrombin time in all patients treated with azithromycin and warfarin concomitantly. Concurrent use of macrolides and warfarin in clinical practice has been associated with increased anticoagulant effects.
- The following drug interactions have not been reported in clinical trials with azithromycin; however, no specific drug interaction studies have been performed to evaluate potential drug-drug interaction. Nonetheless, they have been observed with macrolide products. Until further data are developed regarding drug interactions when azithromycin and these drugs are used concomitantly, careful monitoring of patients is advised:
- Digoxin - elevated digoxin levels. - Ergotamine or dihydroergotamine - acute ergot toxicity characterized by severe peripheral vasospasm and dysesthesia. - Triazolam - Increased pharmacologic effect of triazolam by decreasing the clearance of triazolam. - Drugs metabolized by the cytochrome P450 system – elevations of serum carbamazepine, terfenadine, cyclosporine, hexobarbital, and phenytoin levels. |FDAPregCat=B |useInPregnancyFDA=* Reproduction studies have been performed in rats and mice at doses up to moderately maternally toxic dose levels (i.e., 200 mg/kg/day by the oral route). These doses, based on a mg/m2 basis, are estimated to be 4 and 2 times, respectively, the human daily dose of 500 mg by the oral route. In the animal studies, no evidence of harm to the fetus due to azithromycin was found. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, azithromycin should be used during pregnancy only if clearly needed. |useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Azithromycin (injection) in women who are pregnant. |useInLaborDelivery=There is no FDA guidance on use of Azithromycin (injection) during labor and delivery. |useInNursing=There is no FDA guidance on the use of Azithromycin (injection) with respect to nursing mothers. |useInPed=There is no FDA guidance on the use of Azithromycin (injection) with respect to pediatric patients. |useInGeri=* Pharmacokinetic studies with intravenous azithromycin have not been performed in older volunteers. Pharmacokinetics of azithromycin following oral administration in older volunteers (65-85 years old) were similar to those in younger volunteers (18-40 years old) for the 5-day therapeutic regimen.
- In multiple-dose clinical trials of intravenous azithromycin in the treatment of community-acquired pneumonia, 45% of patients (188/414) were at least 65 years of age and 22% of patients (91/414) were at least 75 years of age. No overall differences in safety were observed between these subjects and younger subjects in terms of adverse events, laboratory abnormalities, and discontinuations. Similar decreases in clinical response were noted in azithromycin- and comparator-treated patients with increasing age.
- Azithromycin for injection contains 114 mg (4.96 mEq) of sodium per vial. At the usual recommended doses, patients would receive 114 mg (4.96 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. The total sodium content from dietary and non-dietary sources may be clinically important with regard to such diseases as congestive heart failure.
|useInGender=There is no FDA guidance on the use of Azithromycin (injection) with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Azithromycin (injection) with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of Azithromycin (injection) in patients with renal impairment. |useInHepaticImpair=There is no FDA guidance on the use of Azithromycin (injection) in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of Azithromycin (injection) in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Azithromycin (injection) in patients who are immunocompromised.
|administration=* Intravenous |monitoring=There is limited information regarding Monitoring of Azithromycin (injection) in the drug label.
|IVCompat=There is limited information regarding IV Compatibility of Azithromycin (injection) in the drug label.
|overdose=There is limited information regarding Chronic Overdose of Azithromycin (injection) in the drug label.
|drugBox=
|mechAction=* * Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and, thus, interfering with microbial protein synthesis. Nucleic acid synthesis is not affected.
- Azithromycin concentrates in phagocytes and fibroblasts as demonstrated by in vitro incubation techniques. Using such methodology, the ratio of intracellular to extra-cellular concentration was >30 after one hour incubation. In vivo studies suggest that concentration in phagocytes may contribute to drug distribution to inflamed tissues.
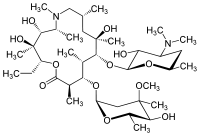
|structure=* Azithromycin for injection contains the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for intravenous injection. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.00. Azithromycin has the following structural formula.
|PD=There is limited information regarding Pharmacodynamics of Azithromycin (injection) in the drug label.
|PK=:

|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Azithromycin (injection) in the drug label.
|clinicalStudies=:
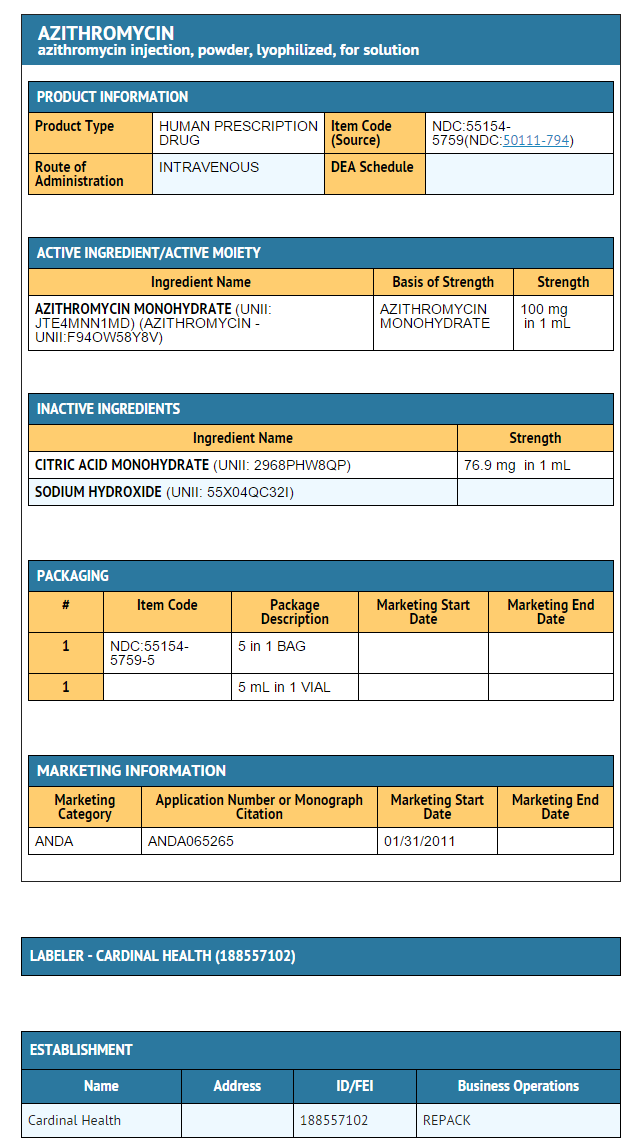
|howSupplied=* Azithromycin for injection is supplied in white to almost white lyophilized form under a vacuum in a 10-mL vial equivalent to 500 mg of azithromycin for intravenous administration. Each vial also contains sodium hydroxide and 413.6 mg citric acid.
- These are packaged as follows:
- 1 x 10 vials of 500 mg
- 1 x 25 vials of 500 mg
|storage=* Store dry powder at 20 to 25°C (68 to 77°F)
|packLabel=

|fdaPatientInfo=There is limited information regarding Patient Counseling Information of Azithromycin (injection) in the drug label.
|alcohol=* Alcohol-Azithromycin (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=* AZITHROMYCIN®[1]
|drugShortage= }} {{#subobject:
|Page Name=Azithromycin (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Azithromycin (injection) |Label Name=Azithromycin (injection)11.png
}}
{{#subobject:
|Label Page=Azithromycin (injection) |Label Name=Azithromycin (injection)11.png
}}
}}