Azapropazone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Rheumox |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 20 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
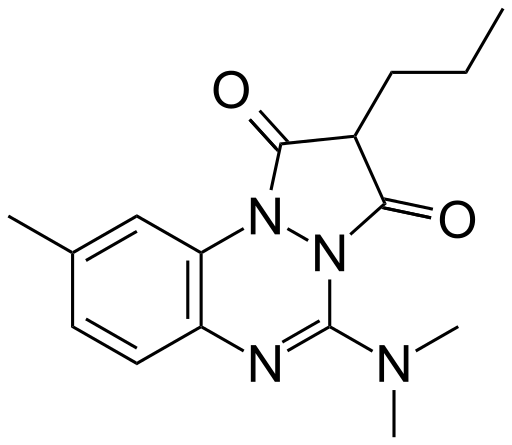
| Formula | C16H20N4O2 |
| Molar mass | 300.36 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Azapropazone |
|
Articles |
|---|
|
Most recent articles on Azapropazone Most cited articles on Azapropazone |
|
Media |
|
Powerpoint slides on Azapropazone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Azapropazone at Clinical Trials.gov Clinical Trials on Azapropazone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Azapropazone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Azapropazone Discussion groups on Azapropazone Patient Handouts on Azapropazone Directions to Hospitals Treating Azapropazone Risk calculators and risk factors for Azapropazone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Azapropazone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Azapropazone is a non-steroidal anti-inflammatory drug. It is manufactured by Goldshield under the tradename Rheumox.[1]
It was available in the UK as a prescription-only drug, with restrictions due to certain contra-indications and side-effects.[2] Azopropazone has now been discontinued in the British National Formulary.
Azapropazone has a half-life of approximately 20 hours in humans and is not extensively metabolized.[3]
References
- ↑ http://home.intekom.com/pharm/cont_eth/rheumox.html
- ↑ http://www.patient.co.uk/showdoc/30002267/
- ↑ Jones CJ (1976). "The pharmacology and pharmacokinetics of azapropazone - a review". Curr Med Res Opin. 4 (1): 3–16. doi:10.1185/03007997609109361. PMID 770078.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Triazines
- Non-steroidal anti-inflammatory drugs
- Lactams
- Drug