Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of an electron in an atom. The region in which an electron may be found around a single atom in a particular energy state can be calculated from this function. The term "orbital" has become known as either the "mathematical function" or the "region" generated with the function.[1] Specifically, atomic orbitals are the possible quantum states of an individual electron in the electron cloud around a single atom, as described by the function.
Classically, the electrons were thought to orbit the atomic nucleus, much like the planets around the Sun (or more accurately, a moth orbiting very quickly around a lamp). Explaining the behavior of the electrons that "orbit" an atom was one of the driving forces behind the development of quantum mechanics. In quantum mechanics, atomic orbitals are described as wave functions over space, indexed by the n, l, and m quantum numbers of the orbital or by the names as used in electron configurations, as shown on the right. As electrons cannot be described as solid particles (as a planet or a moth) in this way, a more accurate analogy would be that of a huge atmosphere, the spatially distributed electron, around a tiny planet which is the atomic nucleus. Hence the term "orbit" was substituted with something else: orbital.
The orbital names (s, p, d, f, g, h,...) are derived from the characteristics of their spectroscopic lines: sharp, principal, diffuse and fundamental, the rest being named in alphabetical order. For mnemonic reasons, some call them spherical & peripheral.
Orbital names
Orbitals are given names in the form:
- <math>X \, \mathrm{type}^y \ </math>
where X is the energy level corresponding to the principal quantum number n, type is a lower-case letter denoting the shape or subshell of the orbital and it corresponds to the angular quantum number l, and y is the number of electrons in that orbital.
For example, the orbital 1s2 (pronounced "one ess two") has two electrons and is the lowest energy level (n = 1) and has an angular quantum number of l = 0. In some cases, the principal quantum number is given a letter associated with it. For n = 1, 2, 3, 4, 5 ....., the letters associated with those numbers are K, L, M, N, O .... respectively.
Formal quantum mechanical definition
In quantum mechanics, the state of an atom, i.e. the eigenstates of the atomic Hamiltonian, is expanded (see configuration interaction expansion and basis (linear algebra)) into linear combinations of anti-symmetrized products (Slater determinants) of one-electron functions. The spatial components of these one-electron functions are called atomic orbitals. (When one considers also their spin component, one speaks of atomic spin orbitals.)
In atomic physics, the atomic spectral lines correspond to transitions (quantum leaps) between quantum states of an atom. These states are labelled by a set of quantum numbers summarized in the term symbol and usually associated to particular electron configurations, i.e. by occupations schemes of atomic orbitals (e.g. 1s2 2s2 2p6 for the ground state of neon -- term symbol: 1S0).
This notation means that the corresponding Slater determinants have a clear higher weight in the configuration interaction expansion. The atomic orbital concept is therefore a key concept for visualizing the excitation process associated to a given transition. For example, one can say for a given transition that it corresponds to the excitation of an electron from an occupied orbital to a given unoccupied orbital. Nevertheless one has to keep in mind that electrons are fermions ruled by Pauli exclusion principle and cannot be distinguished from the other electrons in the atom. Moreover, it sometimes happens that the configuration interaction expansion converges very slowly and that one cannot speak about simple one-determinantal wave function at all. This is the case when electron correlation is large.
Fundamentally, an atomic orbital is a one-electron wavefunction. Don't forget that when thinking about orbitals, we are often bombarded (even if we don't know it) by the Hartree-Fock vision of molecular orbital theory.
Connection to uncertainty relation
After Heisenberg discovered his uncertainty relation it was discovered by men like Pauling and Mulliken that the consequences were that the electron could no longer be considered as in an exact location in its orbital. Rather the electron's position had to be described by a probability distribution. Therefore, the Bohr atom number n for each orbital became known as an n-sphere in the three dimensional atom and was pictured as a probability cloud where the electron surrounded the atom all at once.
This led to the further description by Heisenberg that if a measurement of the electron was not being taken that it could not be described in one particular location but was everywhere in the electron cloud at once. In other words, quantum mechanics cannot give exact results, but only the probabilities for the occurrence of a variety of possible results. Heisenberg went further and said that the path of a moving particle only comes into existence once we observe it. However strange and counter-intuitive this assertion may seem, quantum mechanics does still tell us the location of the electron's orbital, its probability cloud. Heisenberg was speaking of the particle itself, not its orbital which is in a known probability distribution.
It is important to note that although Heisenberg used infinite sets of positions for the electron in his matrices, this does not mean that the electron could be anywhere in the universe.[citation needed] Rather there are several laws that show the electron must be in one localized probability distribution. An electron is described by its energy in Bohr's atom which was carried over to matrix mechanics. Therefore, an electron in a certain n-sphere had to be within a certain range from the nucleus depending upon its energy.[citation needed] This restricts its location. Also, the number of places an electron can be is also called "the number of cells in its phase space". The Uncertainty Principle set a lower limit to how finely one can chop up classical phase space, so the number of places that an electron can be in its orbital becomes finite. An electron's location in an atom is defined to be in its orbital, but stops at the nucleus and before the next n-sphere orbital begins.
Hydrogen-like atoms
The simplest atomic orbitals are those that occur in an atom with a single electron, such as the hydrogen atom. In this case the atomic orbitals are the eigenstates of the hydrogen Hamiltonian. They can be obtained analytically (see hydrogen atom). An atom of any other element ionized down to a single electron is very similar to hydrogen, and the orbitals take the same form.
For atoms with two or more electrons, the governing equations can only be solved with the use of methods of iterative approximation. Orbitals of multi-electron atoms are qualitatively similar to those of hydrogen, and in the simplest models, they are taken to have the same form. For more rigorous and precise analysis, the numerical approximations must be used.
A given (hydrogen-like) atomic orbital is identified by unique values of three quantum numbers: n, l, and ml. The rules restricting the values of the quantum numbers, and their energies (see below), explain the electron configuration of the atoms and the periodic table.
The stationary states (quantum states) of the hydrogen-like atoms are its atomic orbital. However, in general, an electron's behavior is not fully described by a single orbital. Electron states are best represented by time-depending "mixtures" (linear combinations) of multiple orbitals. See Linear combination of atomic orbitals molecular orbital method.
The quantum number n first appeared in the Bohr model. It determines, among other things, the distance of the electron from the nucleus; all electrons with the same value of n lie at the same distance. Modern quantum mechanics confirms that these orbitals are closely related. For this reason, orbitals with the same value of n are said to comprise a "shell". Orbitals with the same value of n and also the same value of l are even more closely related, and are said to comprise a "subshell".
Qualitative characterization
Limitations on the quantum numbers
An atomic orbital is uniquely identified by the values of the three quantum numbers, and each set of the three quantum numbers corresponds to exactly one orbital, but the quantum numbers only occur in certain combinations of values. The rules governing the possible values of the quantum numbers are as follows:
The principal quantum number n is always a positive integer. In fact, it can be any positive integer, but for reasons discussed below, large numbers are seldom encountered. Each atom has, in general, many orbitals associated with each value of n; these orbitals together are sometimes called a shell.
The azimuthal quantum number <math>\ell</math> is a non-negative integer. Within a shell where n is some integer n0, <math>\ell</math> ranges across all (integer) values satisfying the relation <math>0 \le \ell \le n_0-1</math>. For instance, the n = 1 shell has only orbitals with <math>\ell=0</math>, and the n = 2 shell has only orbitals with <math>\ell=0</math>, and <math>\ell=1</math>. The set of orbitals associated with a particular value of <math>\ell</math> are sometimes collectively called a subshell.
The magnetic quantum number <math>m_\ell</math> is also always an integer. Within a subshell where <math>\ell</math> is some integer <math>\ell_0</math>, <math>m_\ell</math> ranges thus: <math>-\ell_0 \le m_\ell \le \ell_0</math>.
The above results may be summarized in the following table. Each cell represents a subshell, and lists the values of <math>m_\ell</math> available in that subshell. Empty cells represent subshells that do not exist.
| <math>l=0</math> | 1 | 2 | 3 | 4 | ... | |
|---|---|---|---|---|---|---|
| <math>n=1</math> | <math>m_l=0</math> | |||||
| 2 | 0 | -1, 0, 1 | ||||
| 3 | 0 | -1, 0, 1 | -2, -1, 0, 1, 2 | |||
| 4 | 0 | -1, 0, 1 | -2, -1, 0, 1, 2 | -3, -2, -1, 0, 1, 2, 3 | ||
| 5 | 0 | -1, 0, 1 | -2, -1, 0, 1, 2 | -3, -2, -1, 0, 1, 2, 3 | -4, -3, -2 -1, 0, 1, 2, 3, 4 | |
| ... | ... | ... | ... | ... | ... | ... |
Subshells are usually identified by their <math>n</math>- and <math>\ell</math>-values. <math>n</math> is represented by its numerical value, but <math>\ell</math> is represented by a letter as follows: 0 is represented by 's', 1 by 'p', 2 by 'd', 3 by 'f', and 4 by 'g'. For instance, one may speak of the subshell with <math>n=2</math> and <math>\ell=0</math> as a '2s subshell'.
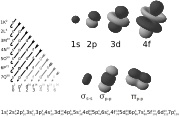
The shapes of orbitals
Any discussion of the shapes of electron orbitals is necessarily imprecise, because a given electron, regardless of which orbital it occupies, can at any moment be found at any distance from the nucleus and in any direction due to the uncertainty principle.
However, the electron is much more likely to be found in certain regions of the atom than in others. Given this, a boundary surface can be drawn so that the electron has a high probability to be found anywhere within the surface, and all regions outside the surface have low values. The precise placement of the surface is arbitrary, but any reasonably compact determination must follow a pattern specified by the behavior of <math>\psi^2</math>, the square of the wavefunction. This boundary surface is what is meant when the "shape" of an orbital is mentioned.
Generally speaking, the number <math>n</math> determines the size and energy of the orbital: as <math>n</math> increases, the size of the orbital increases.
Also in general terms, <math>\ell</math> determines an orbital's shape, and <math>m_\ell</math> its orientation. However, since some orbitals are described by equations in complex numbers, the shape sometimes depends on <math>m_\ell</math> also.
The single <math>s</math>-orbitals (<math>\ell=0</math>) are shaped like spheres. For n=1 the sphere is "solid" (it is most dense at the center and fades exponentially outwardly), but for n=2 or more, each single s-orbital is composed of spherically symmetric surfaces which are nested shells (i.e., the "wave-structure" is radial, following a sinusoidal radial component as well). The <math>s</math>-orbitals for all n numbers are the only orbitals with an anti-node (a region of high wave function density) at the center of the nucleus. All other orbitals (p, d, f, etc.) have angular momentum, and thus avoid the nucleus (having a wave node at the nucleus).
The three <math>p</math>-orbitals have the form of two ellipsoids with a point of tangency at the nucleus (sometimes referred to as a dumbbell). The three <math>p</math>-orbitals in each shell are oriented at right angles to each other, as determined by their respective values of <math>m_\ell</math>.
Four of the five <math>d</math>-orbitals look similar, each with four pear-shaped balls, each ball tangent to two others, and the centers of all four lying in one plane, between a pair of axes. Three of these planes are the <math>xy</math>-, <math>xz</math>-, and <math>yz</math>-planes, and the fourth has the centres on the <math>x</math> and <math>y</math> axes. The fifth and final <math>d</math>-orbital consists of three regions of high probability density: a torus with two pear-shaped regions placed symmetrically on its <math>z</math> axis.
There are seven <math>f</math>-orbitals, each with shapes more complex than those of the <math>d</math>-orbitals.
The shapes of atomic orbitals in one-electron atom are related to 3-dimensional spherical harmonics.
Orbitals table
This table shows all orbital configurations up to 7s, therefore it covers the simple electronic configuration for all elements from the periodic table up to Ununbium (element 112) with the exception of Lawrencium (element 103), which would require a 7p orbital.
Orbital energy
In atoms with a single electron (hydrogen-like atoms), the energy of an orbital (and, consequently, of any electrons in the orbital) is determined exclusively by <math>n</math>. The <math>n=1</math> orbital has the lowest possible energy in the atom. Each successively higher value of <math>n</math> has a higher level of energy, but the difference decreases as <math>n</math> increases. For high <math>n</math>, the level of energy becomes so high that the electron can easily escape from the atom.
In atoms with multiple electrons, the energy of an electron depends not only on the intrinsic properties of its orbital, but also on its interactions with the other electrons. These interactions depend on the detail of its spatial probability distribution, and so the energy levels of orbitals depend not only on <math>n</math> but also on <math>\ell</math>. Higher values of <math>\ell</math> are associated with higher values of energy; for instance, the 2p state is higher than the 2s state. When <math>\ell</math> = 2, the increase in energy of the orbital becomes so large as to push the energy of orbital above the energy of the s-orbital in the next higher shell; when <math>\ell</math> = 3 the energy is pushed into the shell two steps higher.
The energy sequence of the first 24 subshells is given in the following table. Each cell represents a subshell with <math>n</math> and <math>\ell</math> given by its row and column indices, respectively. The number in the cell is the subshell's position in the sequence. Empty cells represent subshells that do not exist.
| <math>s</math> | <math>p</math> | <math>d</math> | <math>f</math> | <math>g</math> | |
|---|---|---|---|---|---|
| 1 | 1 | ||||
| 2 | 2 | 3 | |||
| 3 | 4 | 5 | 7 | ||
| 4 | 6 | 8 | 10 | 13 | |
| 5 | 9 | 11 | 14 | 17 | 21 |
| 6 | 12 | 15 | 18 | 22 | 26 |
| 7 | 16 | 19 | 23 | 27 | 31 |
| 8 | 20 | 24 | 28 | 32 | 36 |
Electron placement and the periodic table
Several rules govern the placement of electrons in orbitals (electron configuration). The first dictates that no two electrons in an atom may have the same set of values of quantum numbers (this is the Pauli exclusion principle). These quantum numbers include the three that define orbitals, as well as s, or spin quantum number. Thus, two electrons may occupy a single orbital, so long as they have different values of <math>s</math>. However, only two electrons, because of their spin, can be associated with each orbital.
Additionally, an electron always tends to fall to the lowest possible energy state. It is possible for it to occupy any orbital so long as it does not violate the Pauli exclusion principle, but if lower-energy orbitals are available, this condition is unstable. The electron will eventually lose energy (by releasing a photon) and drop into the lower orbital. Thus, electrons fill orbitals in the order specified by the energy sequence given above.
This behavior is responsible for the structure of the periodic table. The table may be divided into several rows (called 'periods'), numbered starting with 1 at the top. The presently known elements occupy seven periods. If a certain period has number <math>i</math>, it consists of elements whose outermost electrons fall in the <math>i</math>th shell.
The periodic table may also be divided into several numbered rectangular 'blocks'. The elements belonging to a given block have this common feature: their highest-energy electrons all belong to the same <math>\ell</math>-state (but the <math>n</math> associated with that <math>\ell</math>-state depends upon the period). For instance, the leftmost two columns constitute the 's-block'. The outermost electrons of Li and Be respectively belong to the 2s subshell, and those of Na and Mg to the 3s subshell.
The number of electrons in a neutral atom increases with the atomic number. The electrons in the outermost shell, or valence electrons, tend to be responsible for an element's chemical behavior. Elements that contain the same number of valence electrons can be grouped together and display similar chemical properties.
Element 137
In Classical Quantum Mechanics, any hydrogenoid atom with an atomic number greater than 137 would require 1s electrons to be traveling faster than the speed of light. This makes, however, no sense for two reasons: first of all, in quantum mechanics there is no way for defining the concept "speed". The closest concept is "average momentum", which is not measurable. Secondly, as a system approaches speed of light, the assumptions of classical quantum mechanics fail, and one has to use relativistic quantum mechanics.
Anyhow, the significance of element 137, also known as Untriseptium, was first pointed out by the physicist Richard Feynman. Element 137 is sometimes informally called Feynmanium (symbol Fy).
Since the early 1990’s, physicists have thought that this number (137) might be at the heart of a GUT, or Grand Unified Theory, which could relate the theories of electromagnetism, quantum mechanics, and most especially gravity. A solution to the relationship of Element 137 to a variety of classical constants can be found on the Feynman Website[1], as well as in this discussion of the Fine Structure Constant[2]
See also
- List of Hund's rules
- Electron configuration
- Atomic electron configuration table
- Molecular orbital
- Energy level
References
- ↑ Daintith, J. (2004). Oxford Dictionary of Chemistry. New York: Oxford University Press. ISBN 0-19-860918-3.
- Tipler, Paul (2003). Modern Physics (4 ed.). New York: W. H. Freeman and Company. ISBN 0-7167-4345-0. Unknown parameter
|coauthors=ignored (help)
External links
- Guide to atomic orbitals
- Covalent Bonds and Molecular Structure
- Animation of the time evolution of an hydrogenic orbital
- The Orbitron, a visualization of all common and uncommon atomic orbitals, from 1s to 7g
- Grand table Still images of many orbitals
- David Manthey's Orbital Viewer renders orbitals with n ≤ 30
- Java orbital viewer applet
ar:مدار ذري ca:Orbital atòmic cs:Atomový orbital de:Orbital et:Aatomorbitaal eu:Orbital atomiko fa:اوربیتال gl:Orbital it:Orbitale he:אורביטל אטומי lt:Orbitalė nl:Orbitaal no:Orbital nn:Orbital simple:Atomic orbital sk:Orbitál sl:Orbitala sr:Атомска орбитала sh:Atomska orbitala fi:Atomiorbitaali uk:Атомна орбіталь