Atogepant
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Atogepant is a calcitonin gene-related peptide receptor antagonist that is FDA approved for the prevention of episodic migraines. Common adverse reactions include constipation, fatigue, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Take either 10 mg, 30 mg, or 60 mg of Atogepant orally as prescribed by a doctor.
- Advise patients that Atogepant can either be taken with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atogepant in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atogepant in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Atogepant FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Atogepant in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Atogepant in pediatric patients.
Contraindications
There are no contraindications associated with Atogepant.
Warnings
There is limited information regarding Atogepant Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experiance
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. 1958 patients with migraines received Atogepant to look into the safety of Atogepant usage on patients. All patients in the study received at least one dosage of Atogepant. In the study, 839 patients were exposed to Atogepant for 6 months while 487 patients were exposed to Atogepant for 12 months.
Study 1 and 2
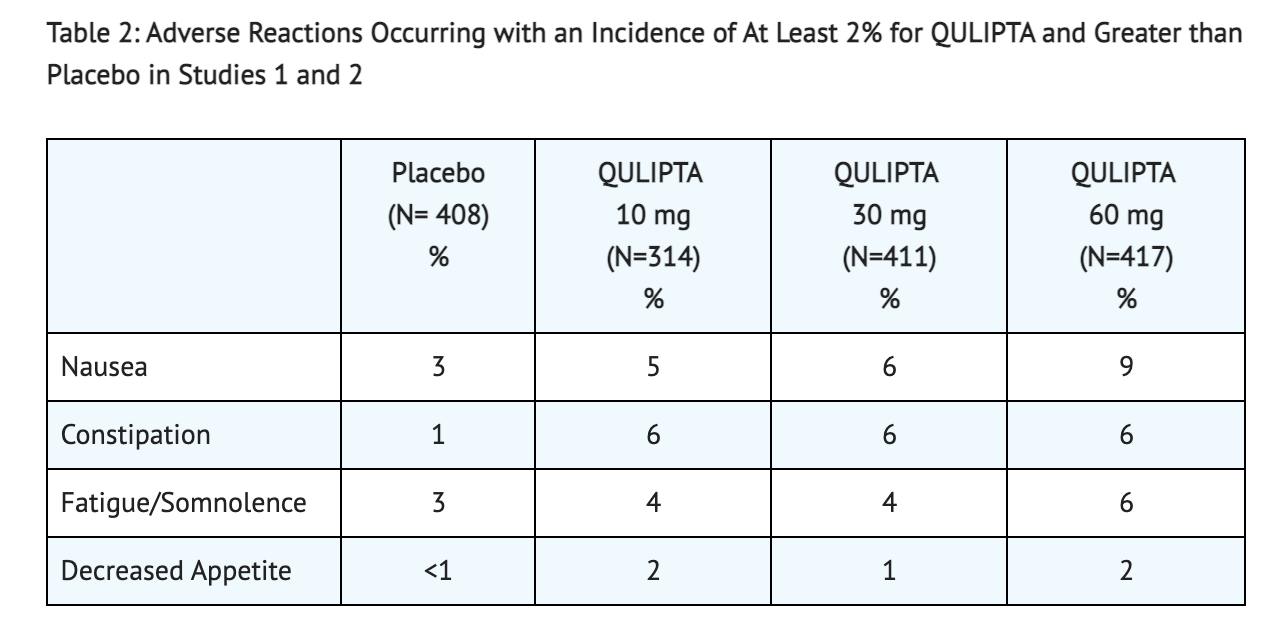
- 12-week, placebo-controlled clinical studies were conducted on patients to look into the adverse reactions observed in patients receiving 10 mg, 30 mg, or 60 mg of Atogepant once daily. 314 patients received 10 mg of Atogepant, 411 patients received 30 mg of Atogepant, 417 patients received 60 mg of Atogepant, and 408 patients received a placebo that had no Atogepant. The patient population was largely Caucasian (80%), and included 88% women. Constipation, nausea, and fatigue were the most common adverse reactions reported in patients (reported at least 4% and greater than placebo). Discontinuation occurred in patients that displayed nausea (0.5%), fatigue (0.5%), and constipation (0.5%).
Table 2 shows the Adverse Reactions found in patients taking Atogepant from Study 1 and Study 2.
Liver Enzyme Elevations
- 1.0% of patients taking Atogepant had a rate of transaminase elevations over 3 times the upper limit of normal. Some of these patients had asymptomatic cases which were ultimately resolved after discontinuation of Atogepant for 8 weeks.
- 1.8% of patients taking the placebo had a rate of transaminase elevations over 3 times the upper limit of normal.
- Jaundice and severe liver injury were not found in patients.
Decreases in Body Weight
- 3.8% of patients taking 10 mg of Atogepant from Study 1 and Study 2 reported a decrease in weight of at least 7%.
- 3.2% of patients taking 30 mg of Atogepant from Study 1 and Study 2 reported a decrease in weight of at least 7%.
- 4.9% of patients taking 60 mg of Atogepant from Study 1 and Study 2 reported a decrease in weight of at least 7%.
- 2.8% of patients taking the placebo from Study 1 and Study 2 reported a decrease in weight of at least 7%.
Postmarketing Experience
There is limited information about "Postmarketing Experiance" in the drug label.
Drug Interactions
CYP3A4 Inhibitors
- Exposure of Atogepant significantly increases with co-administration of Atogepant and itraconazole (a strong CYP3A4 inhibitor).
- 10 mg is recommended dosage of Atogepant when there is concomitant use of Atogepant with a strong CYP3A4 inhibitor.
- Concomitant use of Atogepant with either a moderate or weak CYP3A4 inhibitor requires no change in dosage of Atogepant.
CYP3A4 Inducers
- Exposure of Atogepant significantly decreases with co-administration of Atogepant and a steady state rifampin (a strong CYP3A4 inducer).
- Exposure of Atogepant significantly decreases with concomitant use of Atogepant and moderate inducers of CYP3A4.
- 30 mg or 60 mg is recommended dosage of Atogepant when there is concomitant use of Atogepant with a strong or moderate CYP3A4 inducer.
- Concomitant use of Atogepant with weak CYP3A4 inducer requires no change in dosage of Atogepant.
OATP Inhibitors
- Exposure of Atogepant significantly increases with co-administration of Atogepant and a single dose of rifampin (a OATP inhibitor).
- 10 mg or 30 mg is recommended dosage of Atogepant when there is concomitant use of Atogepant with a OATP inhibitor.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Reproduction studies done on pregnant rabbits showed a rise in adverse developmental effects such as increased incidence of fetal structural variations with increased exposure of Atogepant (0, 30, 90, or 130 mg/kg/day) during the periods of organogenesis, lactation or pregnancy. At 130 mg/kg/day, pregnant rabbits showed an increase in both skeletal variations and fetal visceral. Maternal toxicity was minimal in pregnant rabbits at 130 mg/kg/day. Adverse effects on plasma exposure and embryofetal development was 3 times for humans at the MRHD in pregnant rabbits at 90 mg/kg/day (no-effect dose). Reproduction studies done on pregnant rats showed a decrease in both skeletal ossification and fetal body weight when given either 125 and 750 mg/kg of Atogepant during the period of organogenesis. Adverse effects on plasma exposure and embryofetal development was 4 times for humans at the MRHD in pregnant rats at 15 mg/kg/day (no-effect dose). At 125 mg/kg/day, a decrease in pup body weight was found in rats during periods of gestation and lactation. At 45 mg/kg/day, the no-effect dose, adverse effects of rats on plasma exposure and pre- and postnatal development was 5 times for humans at the MRHD. Advise pregnant, female patients about the potential harms and risks in the embryo when taking Atogepant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Atogepant in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Atogepant during labor and delivery.
Nursing Mothers
No human data on the effects of Atogepant on the breastfed infant and milk production has been conducted. Studies done on lactating rats show Atogepant levels in milk is 2-fold higher than found in milk of the maternal plasma. Advise female patients about both the possible developmental and health benefits as well as potential adverse effects during nursing when taking Atogepant.
Pediatric Use
There is no FDA guidance on the use of Atogepant with respect to pediatric populations.
Geriatic Use
When looking at younger and elderly patients using Atogepant, no clinically significant pharmacokinetic differences were found between both groups of patients. There was not enough patients 65 years or older in age to determine if there are any response differences to Atogepant in comparison to younger patients. Decrease in frequency of decreased renal, cardiac, or hepatic function was found in elderly patients at the low end of the dosing range for Atogepant.
Gender
There is no FDA guidance on the use of Atogepant with respect to specific gender populations.
Race
There is no FDA guidance on the use of Atogepant with respect to specific racial populations.
Renal Impairment
When looking at the clearance of Atogepant, renal route of elimination plays a minor role. 10 mg of Atogepant once daily is the recommended dosage for patients with end-stage renal disease (CLcr <15 mL/min) or patients severe renal impairment (CLcr 15-29 mL/min). Patients with end-stage renal disease who are undergoing intermittent dialysis should start taking Atogepant after dialysis is completed. Patients with mild or moderate renal impairment require no change to dosage usage.
Hepatic Impairment
Patients with mild or moderate hepatic impairment require no change to dosage usage. Patients with severe hepatic impairment should not take Atogepant.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Atogepant in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance on the use of Atogepant with respect to immunocompromised populations.
Administration and Monitoring
Administration
- Take recommended dosage as prescribed by the doctor with or without food.
- Take Atogepant orally and once daily.
Monitoring
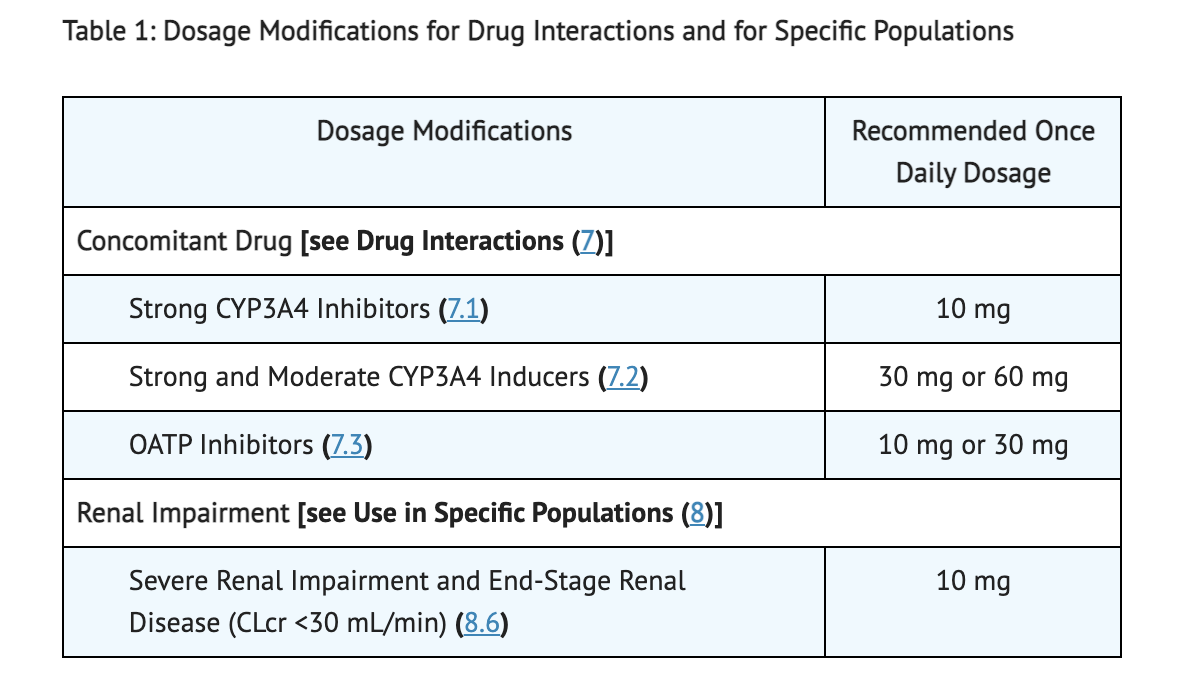
Table 1 shows Dosage Modifications for Concomitant use of Atogepant and Specific Drugs in Patients with Renal Impairment.
IV Compatibility
There is limited information regarding the compatibility of Atogepant and IV administrations.
Overdosage
There is limited information regarding Atogepant overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Atogepant is a calcitonin gene-related peptide receptor antagonist.
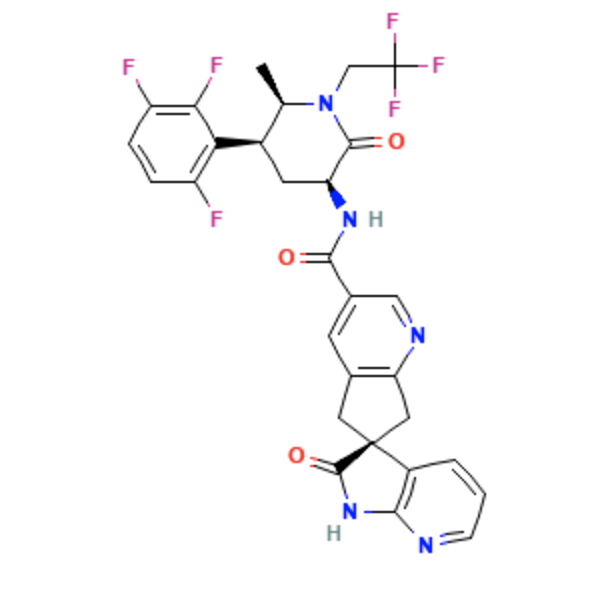
Structure
- Atogepant is a calcitonin gene-related peptide receptor antagonist for oral administration. It has an empirical formula of C29H23F6N5O3 and a molecular weight of 603.5 g/mol.
- The chemical name is (3'S)-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2'-oxo-1',2',5,7- tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxamide.
Pharmacodynamics
Cardiac Electrophysiology
- Atogepant does not prolong the QT interval at 5 times the maximum recommended dosage to any clinically relevant extent.
Pharmacokinetics
Absorption
- The absorption of Atogepant is around 1 to 2 hours with peak plasma concentrations.
- 170 mg is the max dose-proportional pharmacokinetics found in Atogepant with no accumulation.
Effect of Food
- The effect of food was not significant in patients eating a high-fat meal.
- Patients who eat a high-fat meal showed a decrease in the AUC by 18% which resulted in no changes in median time to maximum Atogepant plasma concentration.
- Patients who eat a high-fat meal showed a decrease in the Cmax by 22% which resulted in no changes in median time to maximum Atogepant plasma concentration.
Distribution
- No concentration dependency of the binding of Atogepant to the plasma protein when looking at a range of 0.1 to 10 µM.
- 4.7% is the unbound fraction of Atogepant in human plasma.
- 292 L is the mean apparent volume of distribution for Atogepant.
Elimination
Metabolism
- CYP3A4 plays a primary role in the elimination of Atogepant during metabolism.
- The primary components that make up the human plasma was M23 and Atogepant.
Excretion
- 11 hours is the elimination half-life found in Atogepant.
- 19 L/hr is the mean apparent oral clearance found in Atogepant.
- In feces, after oral administration of 50 mg 14C-Atogepant, 42% of Atogepant was found in which all was found unchanged.
- In urine, after oral administration of 50 mg 14C-Atogepant, 5% of Atogepant was found in which all was found unchanged.
Specific Population
Patients with Renal Impairment
- Renal route of elimination does not play a significant role in Atogepant clearance.
- Pharmacokinetics for patients with mild or moderate renal impairment has shown no significant differences when using Atogepant.
- 10 mg of Atogepant is the recommended dosage for patients either with severe renal impairment or end-stage renal disease.
Patients with Hepatic Impairment
- A 24% increase in total exposure of Atogepant was reported in patients with pre-existing mild hepatic impairment.
- A 15% increase in total exposure of Atogepant was reported in patients with moderate hepatic impairment.
- A 38% increase in total exposure of Atogepant was reported in patients with severe hepatic impairment.
- Patients should avoid Atogepant treatment if they have severe hepatic impairment.
Other Specific Populations
- No dosage changes in Atogepant is required based on a patients sex, race, body weight, or age.
Drug Interactions
In Vitro Studies:
Enzymes
- CYPs 3A4, 2B6, 2C8, 2D6, 1A2, 2C9, and 2C19 are not inhibited by Atogepant.
- UGT1A1 and MAO-A are not inhibited by Atogepant.
- MAO-A, CYP450s, or UGT1A1 inhibition is not clinically significant when looking at Atogepant as a perpetrator of drug-drug interactions.
- CYP2B6, CYP3A4, or CYP1A2 are not induced by Atogepant.
Transporters
- A substrate of OATP1B1, P-gp, OAT1, OATP1B3, and BCRP is Atogepant.
- Concomitant use of OATP inhibitors and Atogepant should have dosage adjustments based on clinical studies.
- Increase in Atogepant exposure is not expected with co-administration of either P-gp inhibitors or BCRP inhibitors and Atogepant.
- OCT2, MATE1, or OAT3 substrate is not Atogepant.
- Transporters such as NTCP, BSEP, BCRP, OAT1, OAT3, MRP4, P-gp, or MRP3 are not inhibited by Atogepant.
- OATP1B3, MATE1, OATP1B1, and OCT1 are weakly inhibited by Atogepant.
In Vivo Studies:
CYP3A4 Inhibitors
- AUC significantly increased by 5.5-fold in exposure of Atogepant with co-administration of Atogepant and itraconazole (a strong CYP3A4 inhibitor)
- Cmax significantly increased by 2.15-fold in exposure of Atogepant with co-administration of Atogepant and itraconazole (a strong CYP3A4 inhibitor).
- A 1.7 fold increase of Atogepant AUC occurred with co-administration of Atogepant and moderate CYP3A4 inhibitors.
- A 1.1 fold increase of Atogepant AUC occurred with co-administration of Atogepant and weak CYP3A4 inhibitors.
- Co-administration of Atogepant with either weak or moderate CYP3A4 inhibitors can create changes in Atogepant exposure which is not clinically significant.
CYP3A4 Inducers
- A 60% decrease in Atogepant AUC was reported in patients with co-administration of Atogepant and rifampin (a strong CYP3A4 inducer).
- A 30% decrease in Atogepant Cmax was reported in patients with co-administration of Atogepant and rifampin (a strong CYP3A4 inducer).
- Decrease in exposure of Atogepant may occur due to moderate inducers of CYP3A4.
- Concomitant use of Atogepant and weak inducers of CYP3A4 are not expected to have a clinically significant interaction.
BCRP/OATP/P-gp Inhibitors
- AUC of Atogepant increased 2.85-fold in patients with co-administration of Atogepant and a single dose of rifampin (an OATP inhibitor).
- Cmax of Atogepant increased 2.23-fold in patients with co-administration of Atogepant and a single dose of rifampin (an OATP inhibitor).
- AUC of Atogepant increased 26% in patients with co-administration of Atogepant and quinidine (a P-gp inhibitor). Exposure of Atogepant is not clinically significant for co-administration of Atogepant and P-gp inhibitors.
- Cmax of Atogepant increased 4% in patients with co-administration of Atogepant and quinidine (a P-gp inhibitor). Exposure of Atogepant is not clinically significant for co-administration of Atogepant and P-gp inhibitors.
- Exposure of Atogepant increased 1.2-fold with co-administration of Atogepant and BCRP inhibitors as reported in PBPK modeling. This increase in exposure of Atogepant may not be clinically significant.
Other Drug Interaction Evaluations
- Pharmacokinetic interactions were not deemed significant in either Atogepant or co-administered oral contraceptive components. Oral contraceptive components include estradiol and levonorgestrel, famotidine, esomeprazole, acetaminophen, naproxen, or sumatriptan.
Nonclinical Toxicology
Carcinogenicity
- No signs of drug-related tumors in either mice (0, 5, 20, or 75 mg/kg/day in males; 0, 5, 30, 160 mg/kg/day in females) or rats (0, 10, 20, or 100 mg/kg in males; 0, 25, 65, or 200 mg/kg in females) that were given different dosages of Atogepant.
- At the highest dosage given in mice, plasma exposure was 8 times higher in mice compared to humans when given 60 mg/day.
- At the highest dosage given in rats, plasma exposure was 20-35 times higher in rats compared to humans when given 60 mg/day.
Mutagenicity
- Vitro and Vivo assays both showed that Atogepant was negative.
Impairment of Fertility
- No adverse reactions were seen in rats reproductive performance or fertility when given either 0, 5, 20, or 125 mg/kg/day of Atogepant.
- At the highest dosage given to rats, plasma exposure was was 15 times higher in rats compared to humans when given 60 mg/day.
Clinical Studies
Study 1
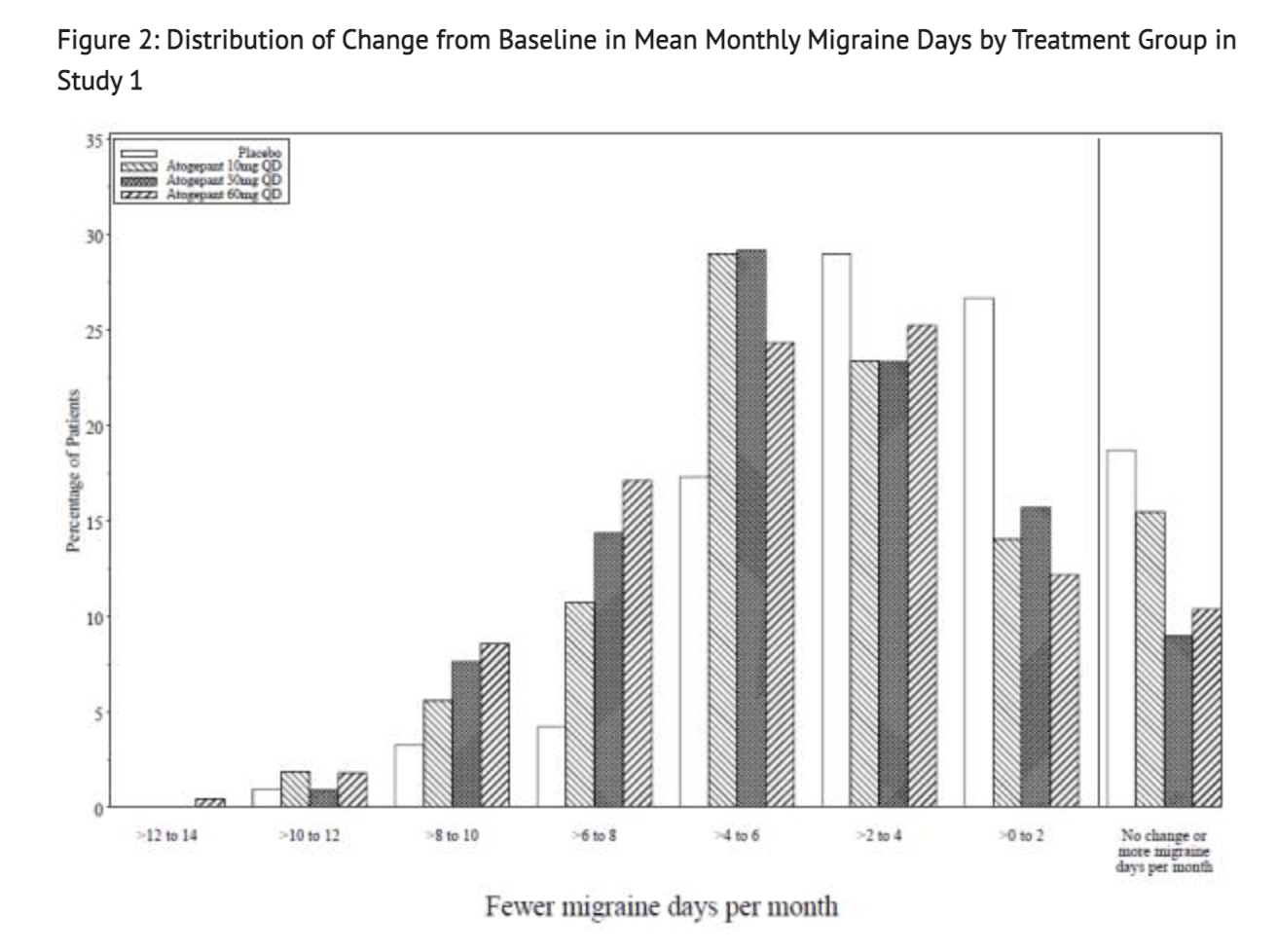
- This study was a randomized, multicenter, double-blind, placebo-controlled study that looked into patients with at least a 1-year history of migraine with or without aura to test the efficacy of Atogepant. Study 1 was a randomized 1:1:1:1 trial where patients either received 10 mg of Atogepant, 30 mg of Atogepant, 60 mg of Atogepant, or a placebo containing no Atogepant. 910 patients made up the study where 222 patients were part of the 10 mg group, 230 patients were part of the 30 mg group, 235 patients were part of the 60 mg group, and 223 patients were part of the placebo group. The study lasted 12 weeks for all patients part of the study. The patient population was largely Caucasian (83%),included 89% women, and had a mean age of 42 years old. Acute headache treatments were allowed for all patients in this study when necessary. Medications that played a role in the CGRP pathway were not permitted. Transient ischemic attacks, myocardial infarction, or strokes found in patients within 6 months prior to screening were not part of this study.
- One goal of Study 1 is to see if there would be change during a 12 week treatment plan to the baseline in mean monthly migraine days. Another goal of this study was to see change from baseline in mean monthly acute medication use days and the change from baseline in mean monthly Activity Impairment in Migraine-Diary as well as Performance of Daily Activities domain scores. The study also looked into the change from baseline in mean monthly headache days and the proportion of patients achieving at least a 50% reduction from baseline in mean monthly migraine days. Finally, the study looked into the change from baseline at Week 12 for Migraine Specific Quality of Life Questionnaire version 2.1 (MSQ v2.1) Role Function-Restrictive (RFR) domain scores.
Table 1 shows the Data Reported from Patients toward Study 1's goals.
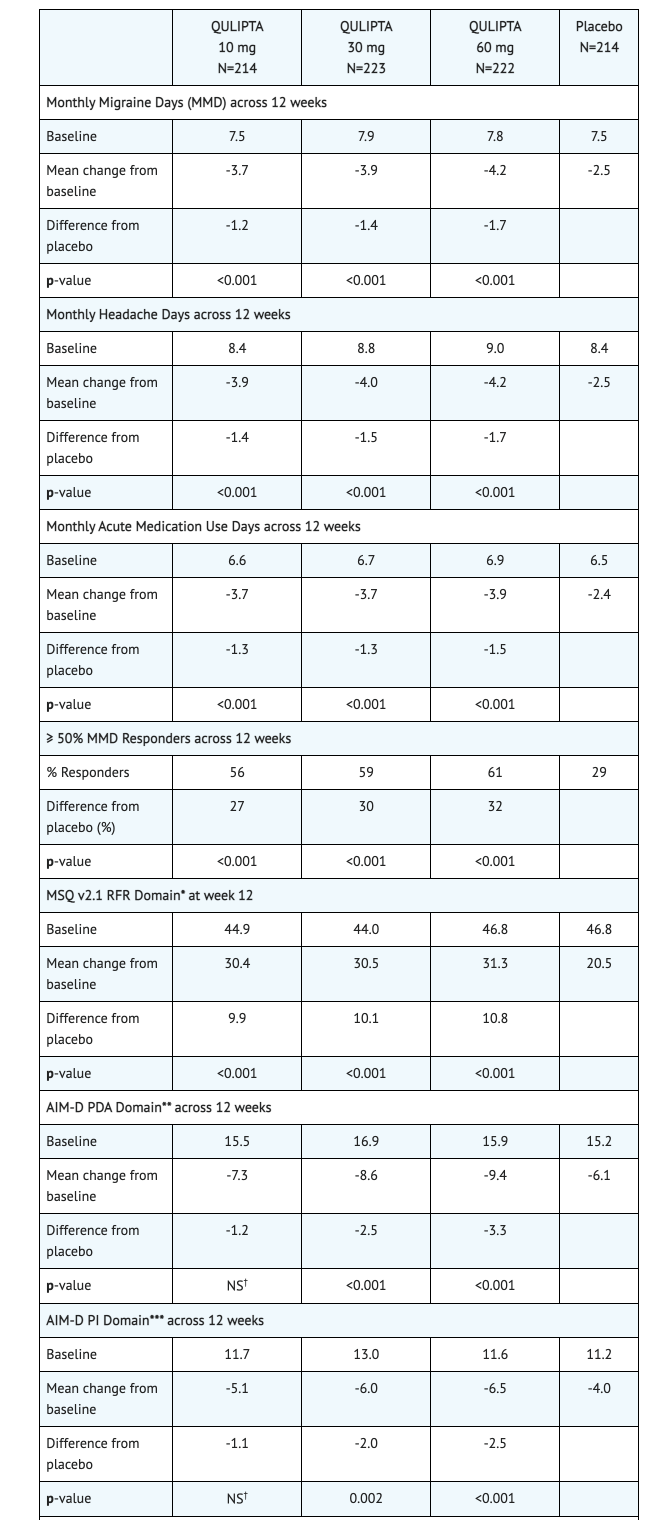
Figure 1 shows Mean Change from Baseline in Mean Monthly Migraine Days.
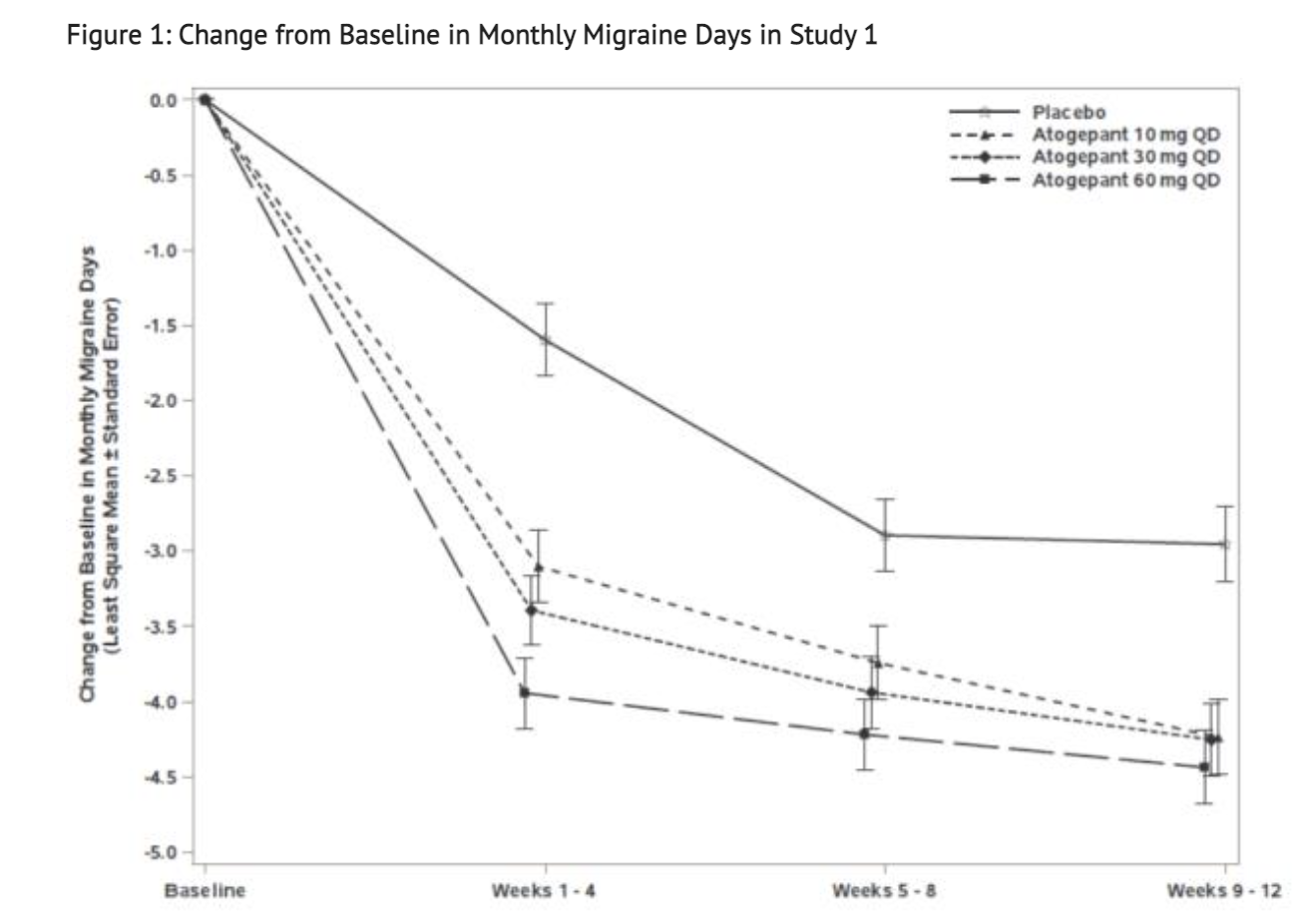
Figure 2 shows Distribution of Change from Baseline in Mean Monthly Migraine Days in the treatment group for this Study.
Study 2
- This study was also a randomized, multicenter, double-blind, placebo-controlled study that looked into patients with at least a 1-year history of migraine with or without aura to test the efficacy of Atogepant. Study 2 was a randomized 1:2:2:2 trial where patients either received 10 mg of Atogepant, 30 mg of Atogepant, 60 mg of Atogepant, or a placebo containing no Atogepant. 652 patients made up the study where 94 patients were part of the 10 mg group, 185 patients were part of the 30 mg group, 187 patients were part of the 60 mg group, and 186 patients were part of the placebo group. The study lasted 12 weeks for all patients part of the study. The patient population was largely Caucasian (76%),included 87% women, and had a mean age of 40 years old. Acute headache treatments were allowed for all patients in this study when necessary. Medications that played a role in the CGRP pathway were not permitted. Transient ischemic attacks, myocardial infarction, or strokes found in patients within 6 months prior to screening were not part of this study.
- The goal of Study 2 was to see change during a 12 week trial from baseline in mean monthly migraine day. 83% patients were able to complete the entirety of the 12 weeks of treatment received during this study. All 3 groups that received any Atogepant showed greater reduction in mean monthly migraine days than the placebo received.
Table 4 shows the Data Reported from Patients toward Study 2's goals.
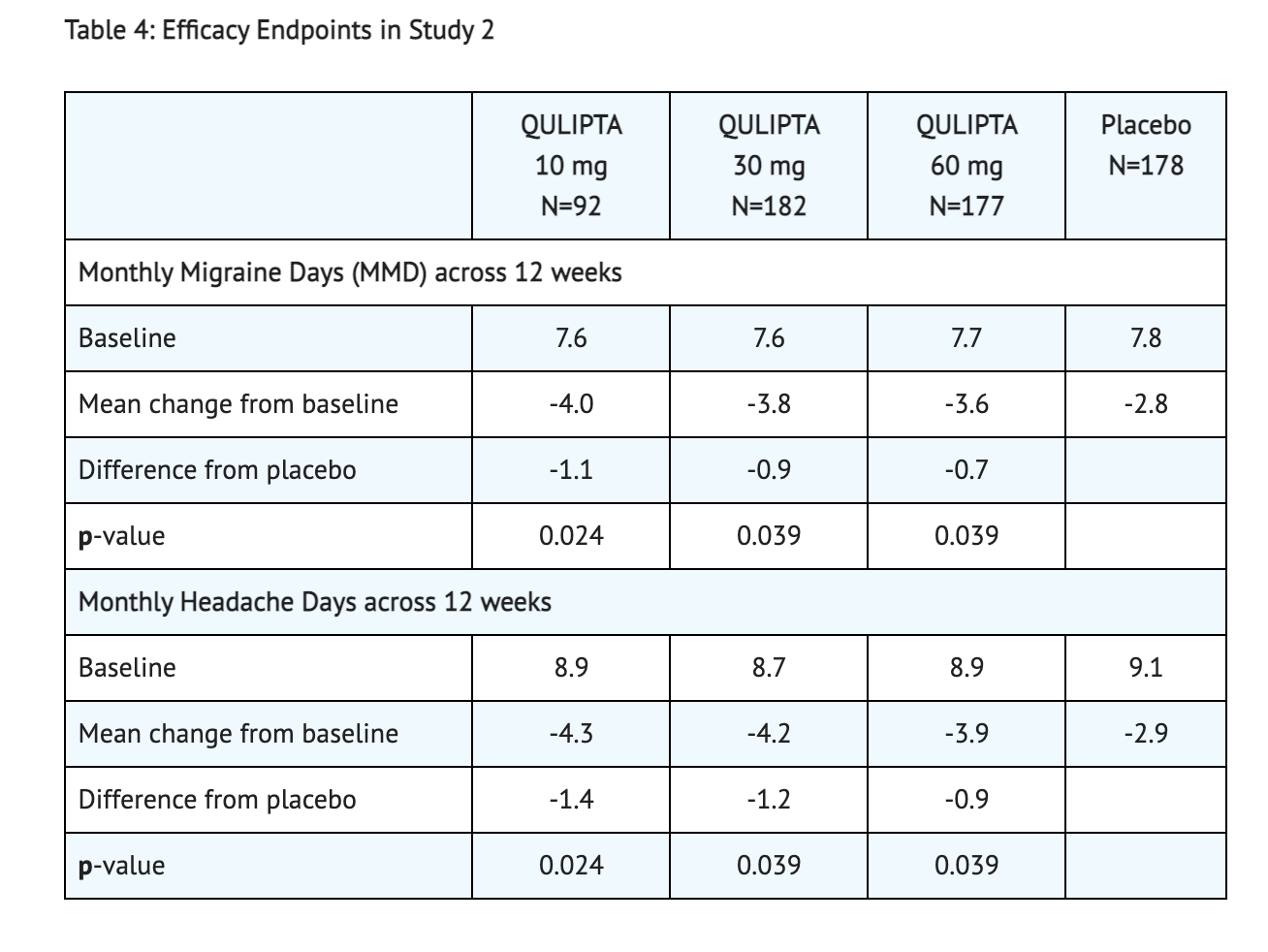
Figure 3 shows The Mean Change from Baseline in Mean Monthly Migraine Days in this Study.
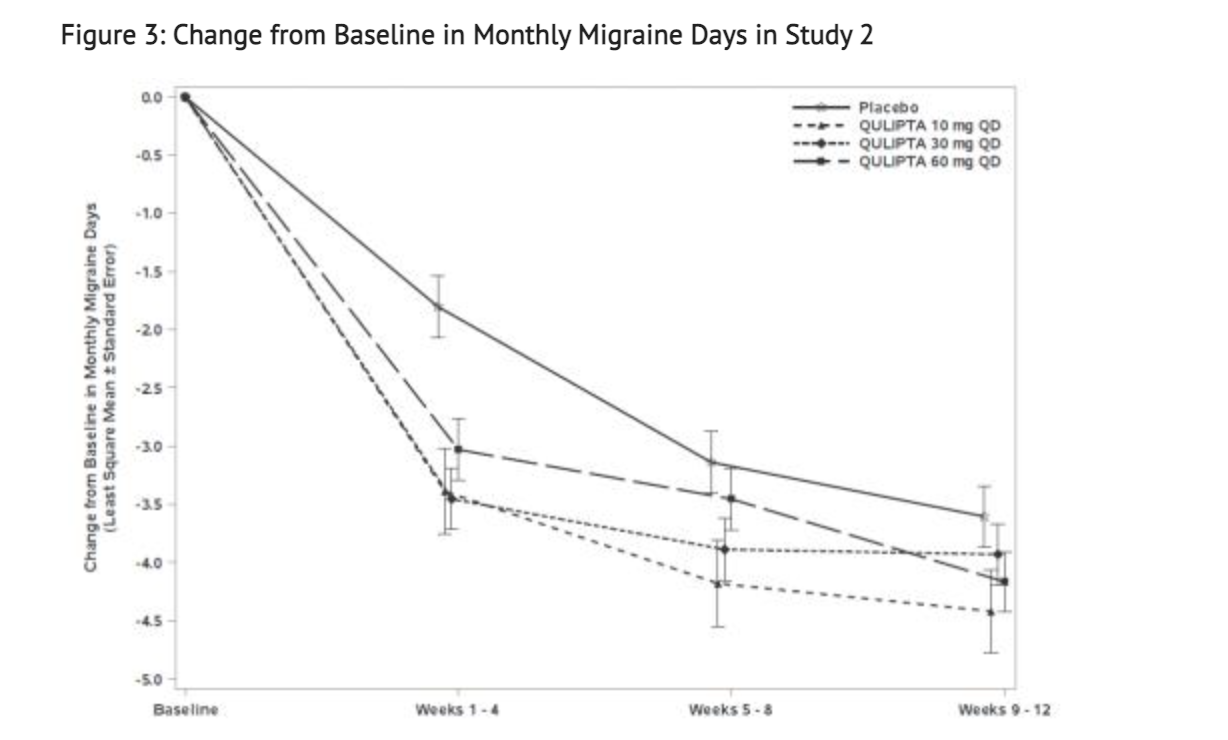
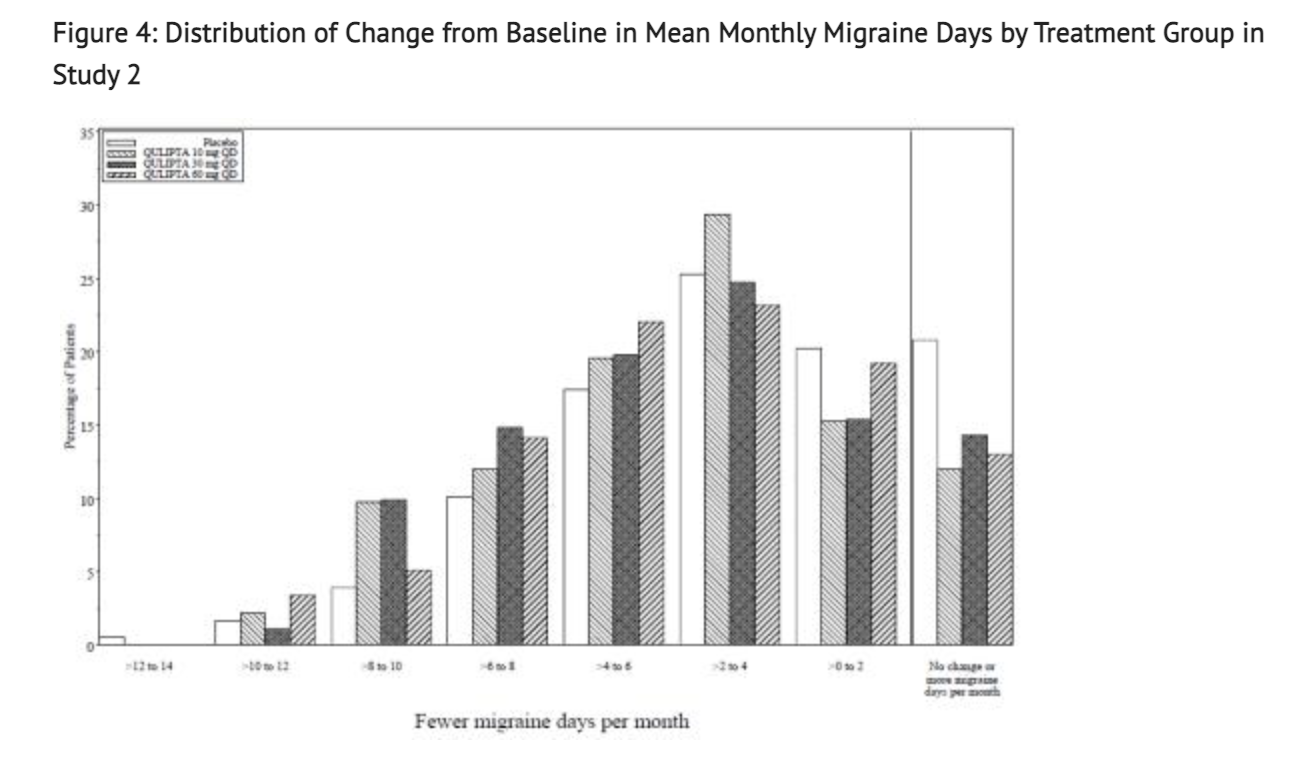
Figure 4 shows the Distribution of Change from Baseline in Mean Monthly Migraine Days in the treatment group for this Study.
How Supplied
- 30 Tablet bottles of 10 mg of Atogepant.
- 30 Tablet bottles of 30 mg of Atogepant.
- 30 Tablet bottles of 60 mg of Atogepant.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Atogepant |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
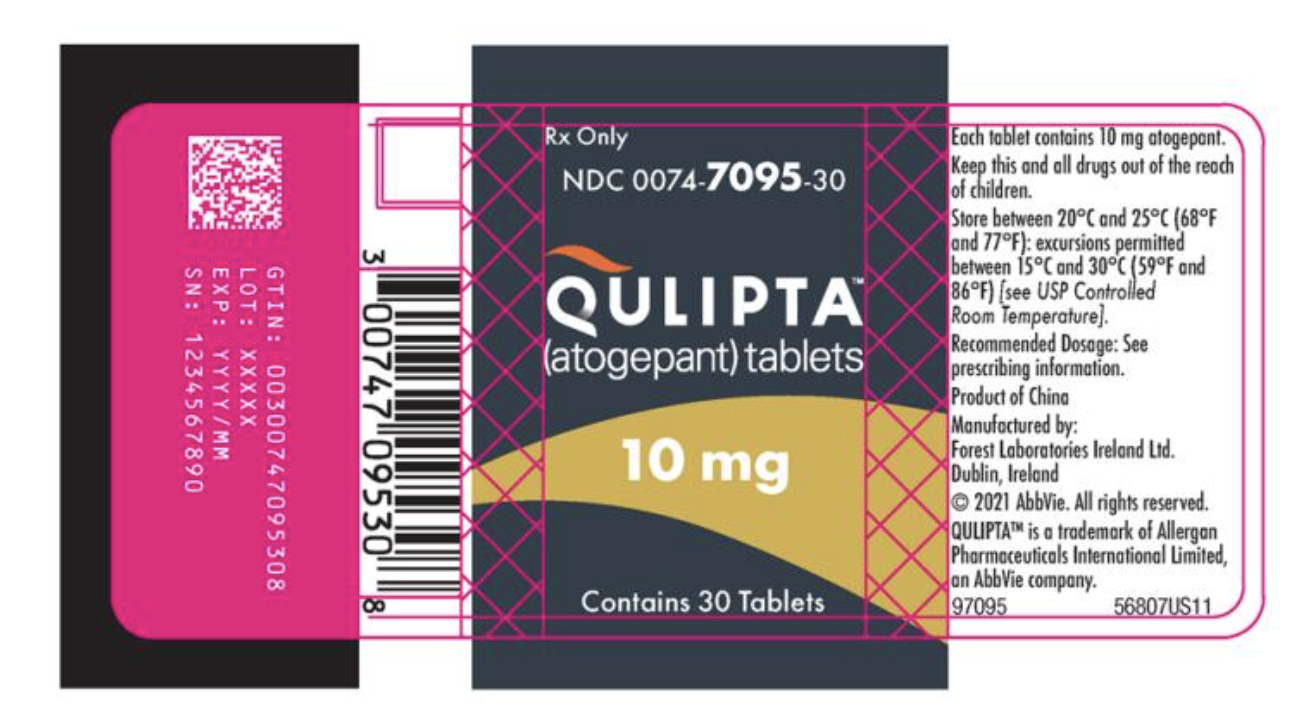
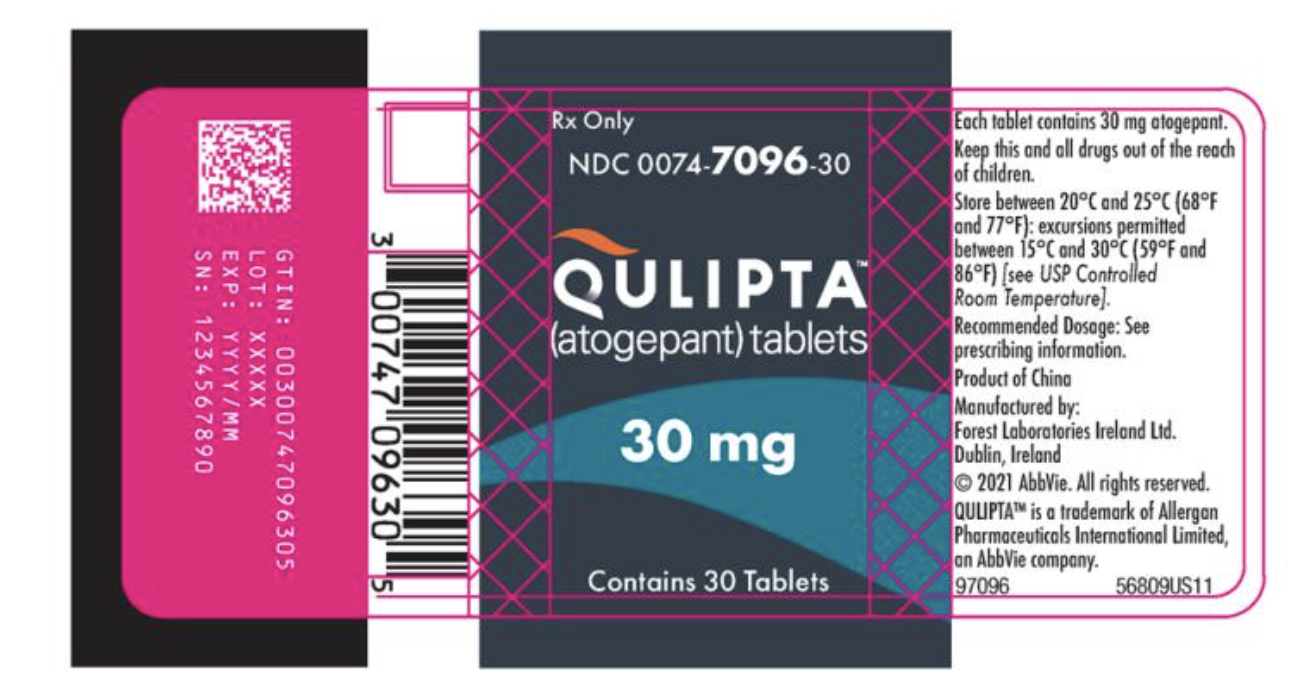

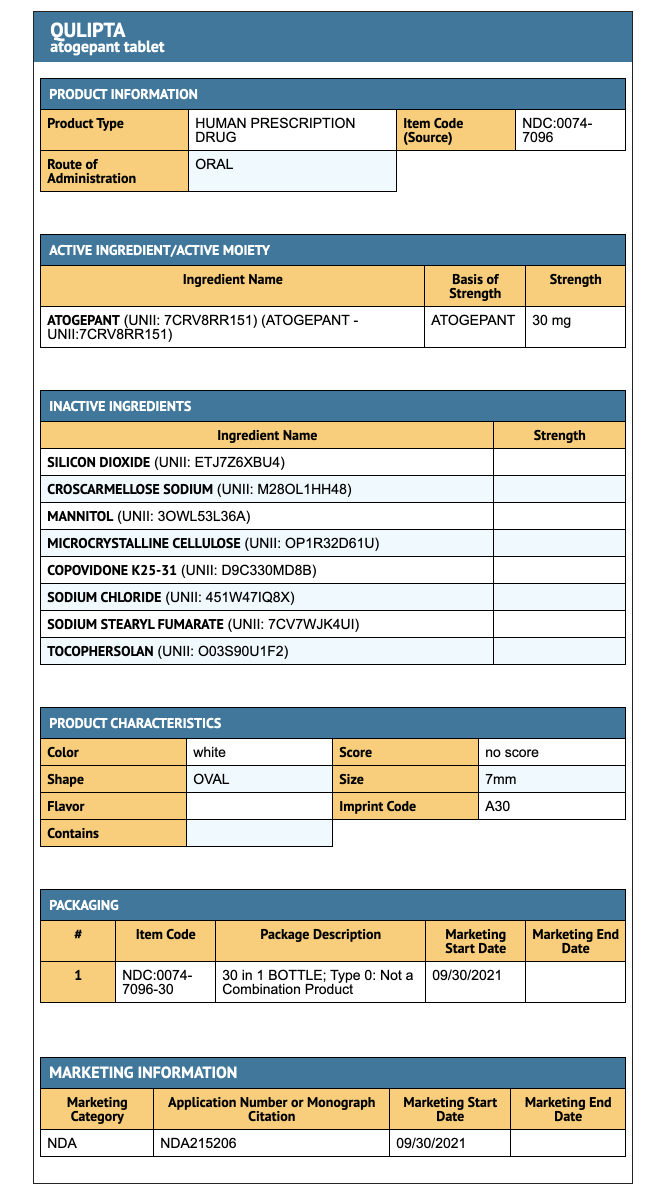
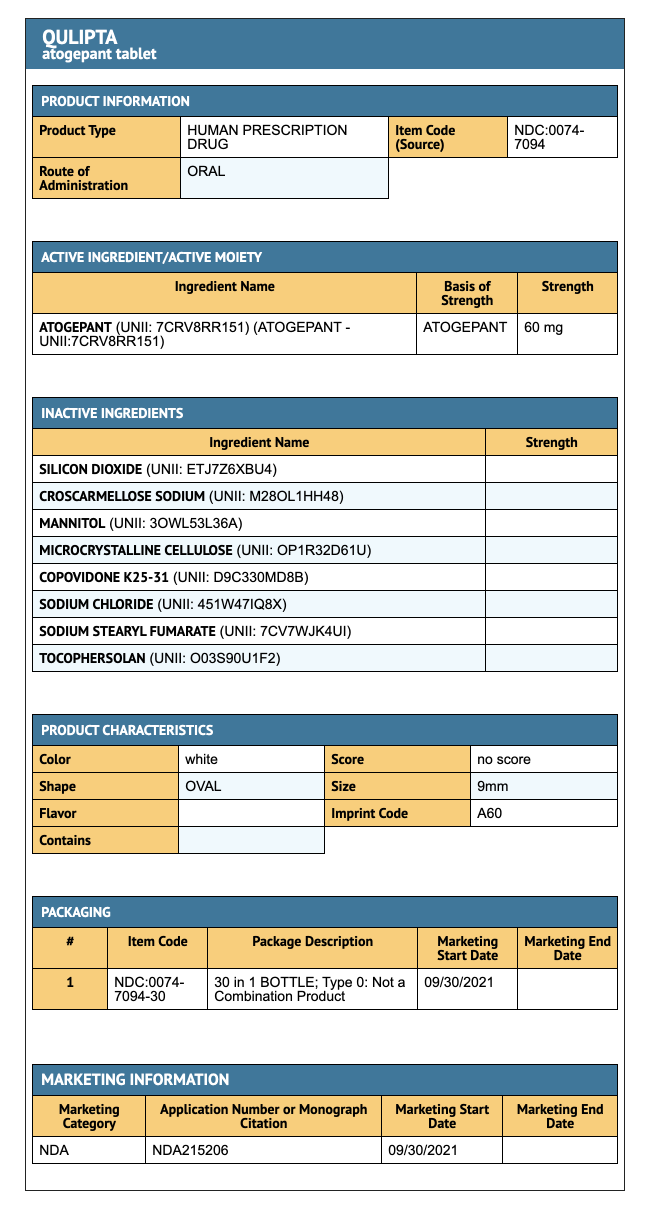
{{#ask: Label Page::Atogepant |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Drug Interactions
- Some drugs that are co-administered with Atogepant may change the dosage amount of Atogepant used in patients.
- Advise patients to report any over-the-counter medications, other prescription medications, grapefruit juice, or herbal products to the prescriber before taking Atogepant.
Atogepant Package Insert:
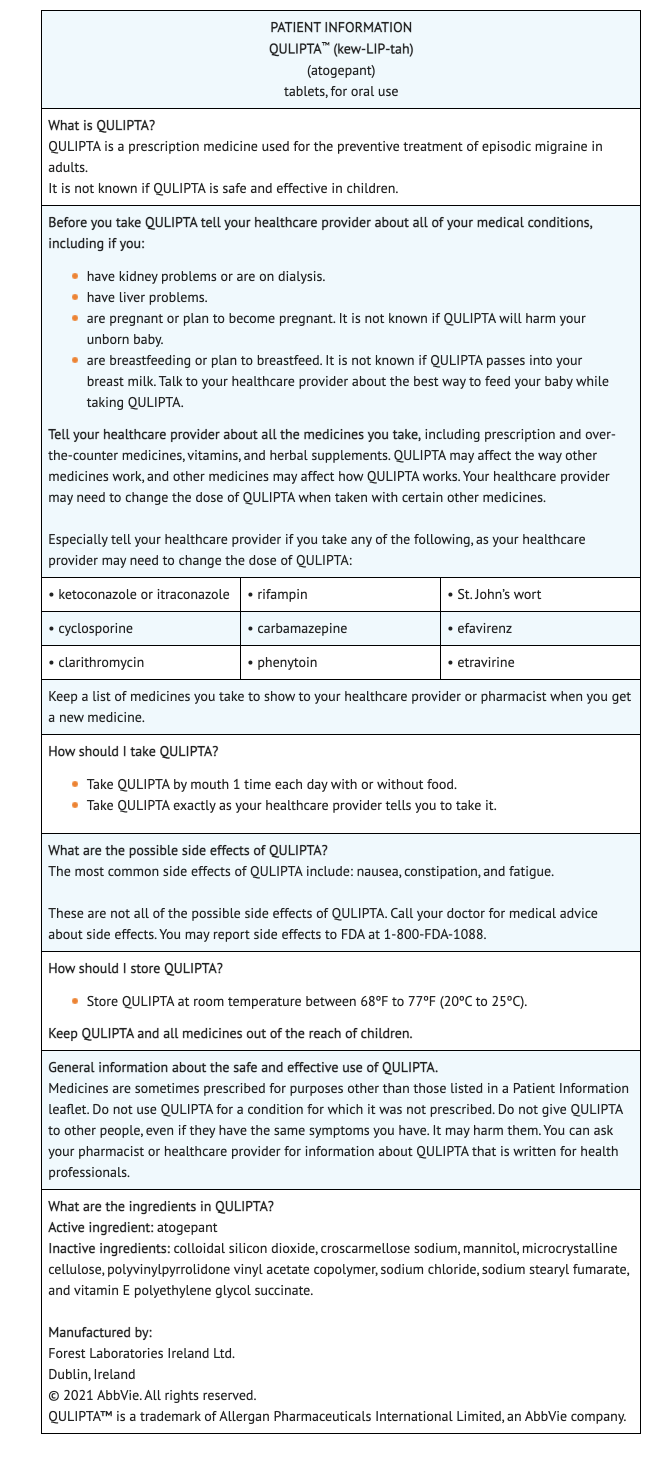
Precautions with Alcohol
Alcohol-Atogepant interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Qulipta
Look-Alike Drug Names
There is limited information regarding Atogepant Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.