Artemether and lumefantrin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Artemether and lumefantrin is an antimanlaric that is FDA approved for the treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum in patients of 5 kg bodyweight and above. Common adverse reactions include headache, anorexia, dizziness, asthenia, arthralgia and myalgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Artemether and lumefantrin FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Artemether and lumefantrin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether and lumefantrin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Artemether and lumefantrin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Artemether and lumefantrin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether and lumefantrin in pediatric patients.
Contraindications
Hypersensitivity
- Known hypersensitivity to artemether, lumefantrine, or to any of the excipients of Coartem Tablets.
Strong CYP3A4 Inducers
- Co-administration of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin and St. John's wort with Coartem Tablets can result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy.
Warnings
Prolongation of the QT Interval
Some antimalarials (e.g., halofantrine, quinine, quinidine) including Coartem Tablets have been associated with prolongation of the QT interval on the electrocardiogram. Coartem Tablets should be avoided in patients:
- With congenital prolongation of the QT interval (e.g., long QT syndrome) or any other clinical condition known to prolong the QTc interval such as patients with a history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease.
- With a family history of congenital prolongation of the QT interval or sudden death.
- With known disturbances of electrolyte balance, e.g., hypokalemia or hypomagnesemia.
- Receiving other medications that prolong the QT interval, such as class IA (quinidine, procainamide, disopyramide), or class III (amiodarone, sotalol) antiarrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents).
- Receiving medications that are metabolized by the cytochrome enzyme CYP2D6 which also have cardiac effects (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Use of QT Prolonging Drugs and Other Antimalarials
- Halofantrine and Coartem Tablets should not be administered within one month of each other due to the long elimination half-life of lumefantrine (3-6 days) and potential additive effects on the QT interval.
- Antimalarials should not be given concomitantly with Coartem Tablets, unless there is no other treatment option, due to limited safety data.
- Drugs that prolong the QT interval, including antimalarials such as quinine and quinidine, should be used cautiously following Coartem Tablets, due to the long elimination half-life of lumefantrine (3-6 days) and the potential for additive effects on the QT interval; ECG monitoring is advised if use of drugs that prolong the QT interval is medically required.
- If mefloquine is administered immediately prior to Coartem Tablets there may be a decreased exposure to lumefantrine, possibly due to a mefloquine-induced decrease in bile production. Therefore, patients should be monitored for decreased efficacy and food consumption should be encouraged while taking Coartem Tablets.
Drug Interactions with CYP3A4
- When Coartem Tablets are co-administered with substrates of CYP3A4 it may result in decreased concentrations of the substrate and potential loss of substrate efficacy. When Coartem Tablets are co-administered with an inhibitor of CYP3A4, including grapefruit juice it may result in increased concentrations of artemether and/or lumefantrine and potentiate QT prolongation. When Coartem Tablets are co-administered with inducers of CYP3A4 it may result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy.
- Drugs that have a mixed effect on CYP3A4, especially antiretroviral drugs such as HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, and those that have an effect on the QT interval should be used with caution in patients taking Coartem Tablets.
- Coartem Tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control.
Drug Interactions with CYP2D6
- Administration of Coartem Tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with Coartem Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Recrudescence
- Food enhances absorption of artemether and lumefantrine following administration of Coartem Tablets.
- Patients who remain averse to food during treatment should be closely monitored as the risk of recrudescence may be greater. In the event of recrudescent P. falciparum infection after treatment with Coartem Tablets, patients should be treated with a different antimalarial drug.
Hepatic and Renal Impairment
- Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment.
Plasmodium vivax Infection
- Coartem Tablets have been shown in limited data (43 patients) to be effective in treating the erythrocytic stage of P. vivax infection. However, relapsing malaria caused by P. vivax requires additional treatment with other antimalarial agents to achieve radical cure i.e., eradicate any hypnozoites forms that may remain dormant in the liver.
Adverse Reactions
Clinical Trials Experience
Serious Adverse Reactions
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rate observed in practice.
The data described below reflect exposure to a 6-dose regimen of Coartem Tablets in 1,979 patients including 647 adults (older than 16 years) and 1,332 children (16 years and younger). For the 6-dose regimen, Coartem Tablets was studied in active-controlled (366 patients) and non-controlled, open-label trials (1,613 patients). The 6-dose Coartem Tablets population was patients with malaria between ages 2 months and 71 years: 67% (1,332) were 16 years and younger and 33% (647) were older than 16 years. Males represented 73% and 53% of the adult and pediatric populations, respectively. The majority of adult patients were enrolled in studies in Thailand, while the majority of pediatric patients were enrolled in Africa.
Tables 1 and 2 show the most frequently reported adverse reactions (вЙ•3%) in adults and children respectively who received the 6-dose regimen of Coartem Tablets. Adverse reactions collected in clinical trials included signs and symptoms at baseline but only treatment emergent adverse events, defined as events that appeared or worsened after the start of treatment, are presented below. In adults, the most frequently reported adverse reactions were headache, anorexia, dizziness, and asthenia. In children, the adverse reactions were pyrexia, cough, vomiting, anorexia, and headache. Most adverse reactions were mild, did not lead to discontinuation of study medication, and resolved.
In limited comparative studies, the adverse reaction profile of Coartem Tablets appeared similar to that of another antimalarial regimen.
Discontinuation of Coartem Tablets due to adverse drug reactions occurred in 1.1% of patients treated with the 6-dose regimen overall: 0.2% (1/647) in adults and 1.6% (21/1,332) in children.
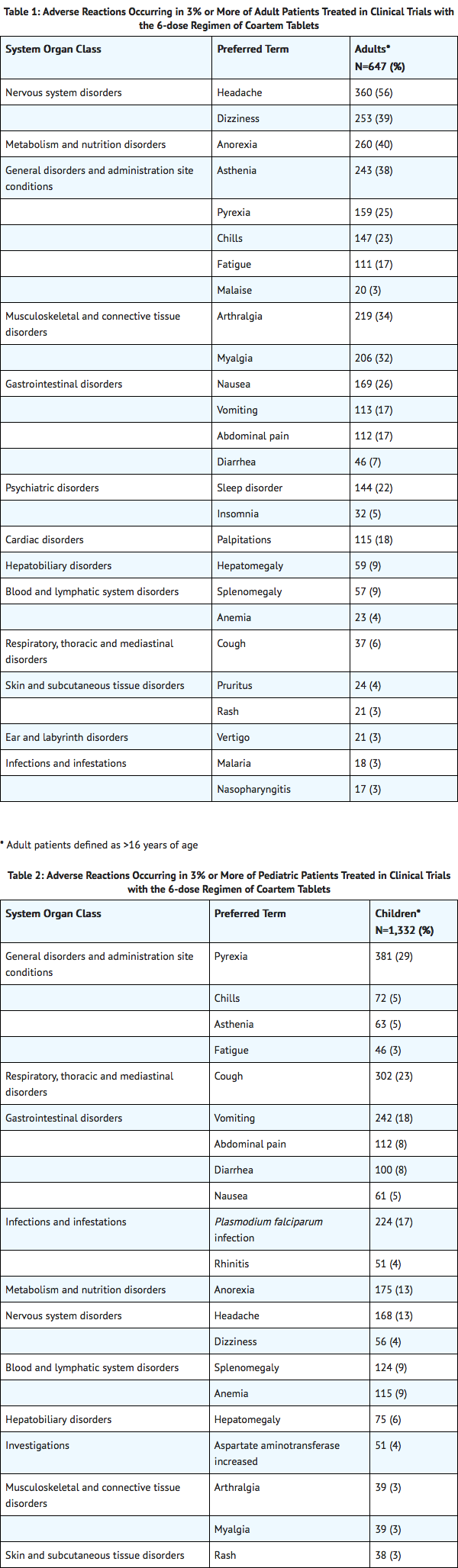
Clinically significant adverse reactions reported in adults and/or children treated with the 6-dose regimen of Coartem Tablets which occurred in clinical studies at <3% regardless of causality are listed below:
- Blood and lymphatic system disorders: eosinophilia
- Ear and labyrinth disorders: tinnitus
- Eye disorders: conjunctivitis
- Gastrointestinal disorders: constipation, dyspepsia, dysphagia, peptic ulcer
- General disorders: gait disturbance
- Infections and infestations: abscess, acrodermatitis, bronchitis, ear infection, gastroenteritis, helminthic infection, hookworm infection, impetigo, influenza, lower respiratory tract infection, malaria, nasopharyngitis, oral herpes, pneumonia, respiratory tract infection, subcutaneous abscess, upper respiratory tract infection, urinary tract infection
Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, hematocrit
decreased, lymphocyte morphology abnormal, platelet count decreased, platelet count increased, white
blood cell count decreased, white blood cell count increased
Metabolism and nutrition disorders: hypokalemia
Musculoskeletal and connective tissue disorders: back pain
Nervous system disorders: ataxia, clonus, fine motor delay, hyperreflexia, hypoaesthesia, nystagmus,
tremor
Psychiatric disorders: agitation, mood swings
Renal and urinary disorders: hematuria, proteinuria
Respiratory, thoracic and mediastinal disorders: asthma, pharyngo-laryngeal pain
Skin and subcutaneous tissue disorders: urticaria
Postmarketing Experience
There is limited information regarding Artemether and lumefantrin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Artemether and lumefantrin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Artemether and lumefantrin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Artemether and lumefantrin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Artemether and lumefantrin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Artemether and lumefantrin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Artemether and lumefantrin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Artemether and lumefantrin in geriatric settings.
Gender
There is no FDA guidance on the use of Artemether and lumefantrin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Artemether and lumefantrin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Artemether and lumefantrin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Artemether and lumefantrin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Artemether and lumefantrin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Artemether and lumefantrin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Artemether and lumefantrin Administration in the drug label.
Monitoring
There is limited information regarding Artemether and lumefantrin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Artemether and lumefantrin and IV administrations.
Overdosage
There is limited information regarding Artemether and lumefantrin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Artemether and lumefantrin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Artemether and lumefantrin Mechanism of Action in the drug label.
Structure
There is limited information regarding Artemether and lumefantrin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Artemether and lumefantrin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Artemether and lumefantrin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Artemether and lumefantrin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Artemether and lumefantrin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Artemether and lumefantrin How Supplied in the drug label.
Storage
There is limited information regarding Artemether and lumefantrin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Artemether and lumefantrin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Artemether and lumefantrin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Artemether and lumefantrin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Artemether and lumefantrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Artemether and lumefantrin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Artemether and lumefantrin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.