Apalutamide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 285: | Line 285: | ||
|PK=(Description) | |PK=(Description) | ||
|nonClinToxic=(Description) | |nonClinToxic=(Description) | ||
|clinicalStudies | |clinicalStudies= | ||
( | *SPARTAN (NCT01946204) was a multicenter, double-blind, randomized (2:1), placebo-controlled clinical trial in which 1207 patients with NM-CRPC were randomized (2:1) to receive either ERLEADA orally at a dose of 240 mg once daily (N = 806) or placebo once daily (N = 401). All patients in the SPARTAN trial received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. Patients were stratified by Prostate Specific Antigen (PSA) Doubling Time (PSADT), the use of bone-sparing agents, and locoregional disease. Patients were required to have a PSADT ≤ 10 months and confirmation of non-metastatic disease by blinded independent central review (BICR). PSA results were blinded and were not used for treatment discontinuation. Patients randomized to either arm discontinued treatment for radiographic disease progression confirmed by BICR, locoregional-only progression, initiation of new treatment, unacceptable toxicity, or withdrawal. | ||
== | *The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 74 years (range 48–97) and 26% of patients were 80 years of age or older. The racial distribution was 66% Caucasian, 12% Asian, and 6% Black. Seventy-seven percent (77%) of patients in both treatment arms had prior surgery or radiotherapy of the prostate. A majority of patients had a Gleason score of 7 or higher (78%). Fifteen percent (15%) of patients had <2 cm pelvic lymph nodes at study entry. Seventy-three percent (73%) of patients received prior treatment with an anti-androgen; 69% of patients received bicalutamide and 10% of patients received flutamide. All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry. Among the patients who discontinued study treatment (N = 279 for placebo and N = 314 for ERLEADA), a greater proportion (80%) of patients treated with placebo received subsequent therapy compared to patients treated with ERLEADA (56%). Locoregional-only progression occurred in 2% of patients overall. | ||
( | *The major efficacy outcome measure of the study was metastasis-free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis, defined as new bone or soft tissue lesions or enlarged lymph nodes above the iliac bifurcation, or death due to any cause, whichever occurred first. Additional efficacy endpoints were time to metastasis (TTM), progression-free survival (PFS) which also includes locoregional progression, time to symptomatic progression, and overall survival (OS). | ||
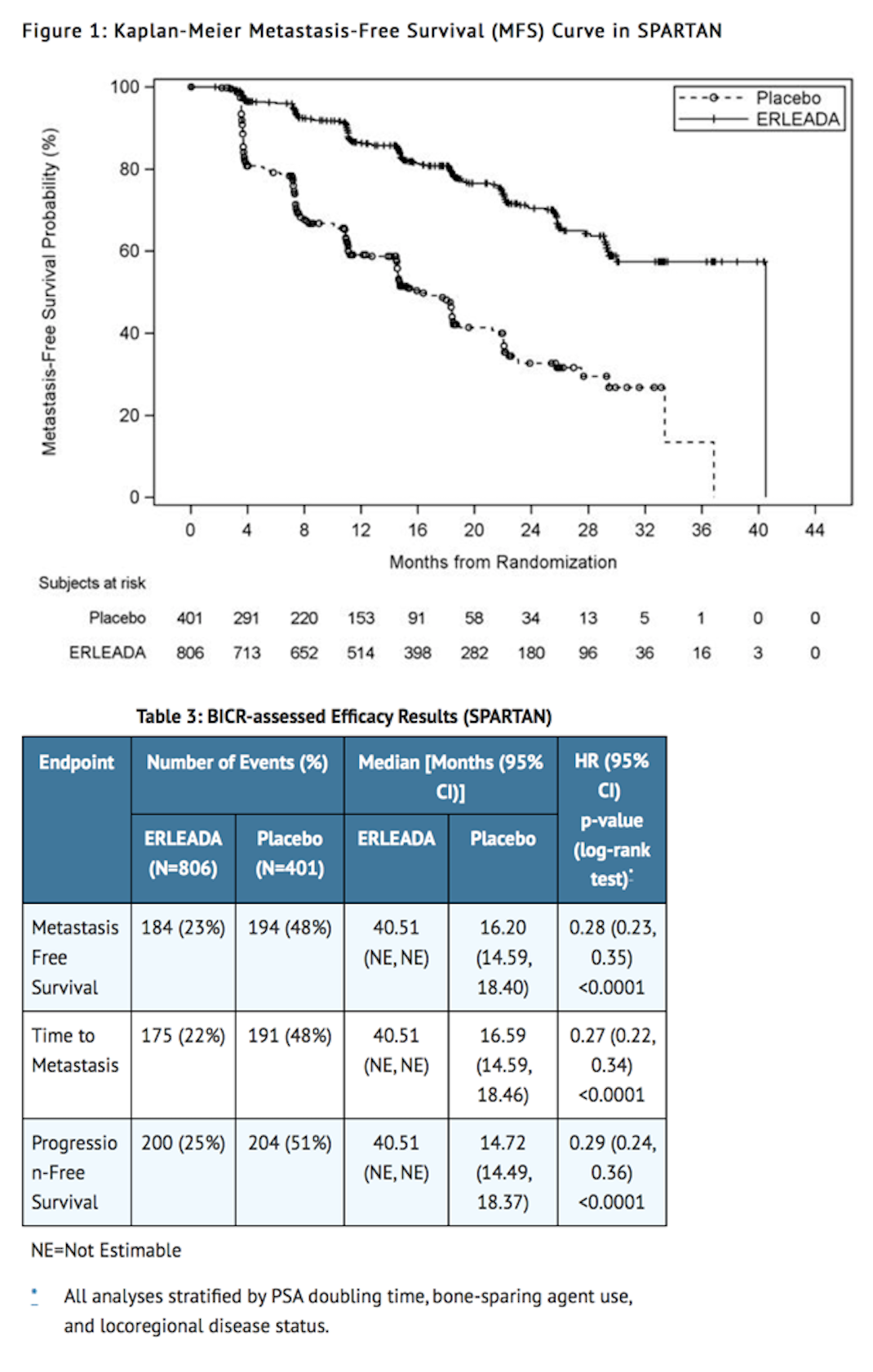
*A statistically significant improvement in MFS was demonstrated in patients randomized to receive ERLEADA compared with patients randomized to receive placebo. Consistent results were observed across patient subgroups including PSADT (≤ 6 months or > 6 months), use of a prior bone-sparing agent (yes or no), and locoregional disease (N0 or N1). The major efficacy outcome was supported by statistically significant improvements in TTM, PFS, and time to symptomatic progression. Overall survival (OS) data were not mature at the time of final MFS analysis (24% of the required number of events). The efficacy results of MFS, TTM, and PFS from SPARTAN are summarized in Figure 1 and Table 3. | |||
[[image:Apalutamide_Clinical_Studies_Figure_and_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|howSupplied=*ERLEADA (apalutamide) 60 mg film-coated tablets are slightly yellowish to greyish green, oblong-shaped tablets debossed with "AR 60" on one side. ERLEADA 60 mg tablets are available in bottles of 120 tablets. Each bottle contains silica gel desiccant. | |howSupplied=*ERLEADA (apalutamide) 60 mg film-coated tablets are slightly yellowish to greyish green, oblong-shaped tablets debossed with "AR 60" on one side. ERLEADA 60 mg tablets are available in bottles of 120 tablets. Each bottle contains silica gel desiccant. | ||
Revision as of 03:01, 25 June 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Apalutamide is an androgen receptor inhibitor that is FDA approved for the treatment of non-metastatic castration-resistant prostate cancer. Common adverse reactions include fatigue, hypertension, rash, diarrhea, nausea, weight decreased, arthralgia, fall, hot flush, decreased appetite, fracture, and peripheral edema.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
Pregnancy
- ERLEADA can cause fetal harm and potential loss of pregnancy.
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Apalutamide in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Apalutamide and IV administrations.
Overdosage
- There is no known specific antidote for apalutamide overdose. In the event of an overdose, stop ERLEADA, undertake general supportive measures until clinical toxicity has been diminished or resolved.
Pharmacology
Apalutamide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
- SPARTAN (NCT01946204) was a multicenter, double-blind, randomized (2:1), placebo-controlled clinical trial in which 1207 patients with NM-CRPC were randomized (2:1) to receive either ERLEADA orally at a dose of 240 mg once daily (N = 806) or placebo once daily (N = 401). All patients in the SPARTAN trial received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. Patients were stratified by Prostate Specific Antigen (PSA) Doubling Time (PSADT), the use of bone-sparing agents, and locoregional disease. Patients were required to have a PSADT ≤ 10 months and confirmation of non-metastatic disease by blinded independent central review (BICR). PSA results were blinded and were not used for treatment discontinuation. Patients randomized to either arm discontinued treatment for radiographic disease progression confirmed by BICR, locoregional-only progression, initiation of new treatment, unacceptable toxicity, or withdrawal.
- The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 74 years (range 48–97) and 26% of patients were 80 years of age or older. The racial distribution was 66% Caucasian, 12% Asian, and 6% Black. Seventy-seven percent (77%) of patients in both treatment arms had prior surgery or radiotherapy of the prostate. A majority of patients had a Gleason score of 7 or higher (78%). Fifteen percent (15%) of patients had <2 cm pelvic lymph nodes at study entry. Seventy-three percent (73%) of patients received prior treatment with an anti-androgen; 69% of patients received bicalutamide and 10% of patients received flutamide. All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry. Among the patients who discontinued study treatment (N = 279 for placebo and N = 314 for ERLEADA), a greater proportion (80%) of patients treated with placebo received subsequent therapy compared to patients treated with ERLEADA (56%). Locoregional-only progression occurred in 2% of patients overall.
- The major efficacy outcome measure of the study was metastasis-free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis, defined as new bone or soft tissue lesions or enlarged lymph nodes above the iliac bifurcation, or death due to any cause, whichever occurred first. Additional efficacy endpoints were time to metastasis (TTM), progression-free survival (PFS) which also includes locoregional progression, time to symptomatic progression, and overall survival (OS).
- A statistically significant improvement in MFS was demonstrated in patients randomized to receive ERLEADA compared with patients randomized to receive placebo. Consistent results were observed across patient subgroups including PSADT (≤ 6 months or > 6 months), use of a prior bone-sparing agent (yes or no), and locoregional disease (N0 or N1). The major efficacy outcome was supported by statistically significant improvements in TTM, PFS, and time to symptomatic progression. Overall survival (OS) data were not mature at the time of final MFS analysis (24% of the required number of events). The efficacy results of MFS, TTM, and PFS from SPARTAN are summarized in Figure 1 and Table 3.
How Supplied
- ERLEADA (apalutamide) 60 mg film-coated tablets are slightly yellowish to greyish green, oblong-shaped tablets debossed with "AR 60" on one side. ERLEADA 60 mg tablets are available in bottles of 120 tablets. Each bottle contains silica gel desiccant.
- NDC Number is 59676-600-12
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
- Store in the original package. Do not discard desiccant. Protect from light and moisture.
Images
Drug Images
{{#ask: Page Name::Apalutamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Apalutamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Apalutamide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Apalutamide Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.