Amiloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
HYPERKALEMIA
See full prescribing information for complete Boxed Warning.
* Like other potassium-conserving agents, amiloride may cause hyperkalemia (serum potassium levels greater than 5.5 mEq per liter) which, if uncorrected, is potentially fatal. Hyperkalemia occurs commonly (about 10%) when amiloride is used without a kaliuretic diuretic. This incidence is greater in patients with renal impairment, diabetes mellitus (with or without recognized renal insufficiency), and in the elderly. When amiloride is used concomitantly with a thiazide diuretic in patients without these complications, the risk of hyperkalemia is reduced to about 1-2 percent. It is thus essential to monitor serum potassium levels carefully in any patient receiving amiloride, particularly when it is first introduced, at the time of diuretic dosage adjustments, and during any illness that could affect renal function.
|
Overview
Amiloride is a potassium-sparing diuretic that is FDA approved for the {{{indicationType}}} of congestive heart failure or hypertension adjunctive with thiazide diuretics or other kaliuretic-diuretic agents. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, diarrhea, loss of appetite, nausea, vomiting, asthenia, cramp, dizziness, headache, cough, and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Congestive Heart Failure or Hypertension
- Dosing Information
- 5 mg tablet daily, should be added to the usual antihypertensive or diuretic dosage of a kaliuretic diuretic.
- The dosage may be increased to 10 mg per day, if necessary.
- More than two 5 mg tablets of amiloride daily usually are not needed, and there is little controlled experience with such doses.
- If persistent hypokalemia is documented with 10 mg, the dose can be increased to 15 mg, then 20 mg, with careful monitoring of electrolytes.
- In treating patients with congestive heart failure after an initial diuresis has been achieved, potassium loss may also decrease and the need for amiloride should be re-evaluated.
- Dosage adjustment may be necessary. Maintenance therapy may be on an intermittent basis.
- If it is necessary to use amiloride alone, the starting dosage should be one 5 mg tablet daily. This dosage may be increased to 10 mg per day, if necessary.
- More than two 5 mg tablets usually are not needed, and there is little controlled experience with such doses. If persistent hypokalemia is documented with 10 mg, the dose can be increased to 15 mg, then 20 mg, with careful monitoring of electrolytes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amiloride in adult patients.
Non–Guideline-Supported Use
Edema, Associated with Thiazolidinedione Use
- Dosing Information
- 10 mg once daily[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amiloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amiloride in pediatric patients.
Contraindications
- Amiloride should not be used in the presence of elevated serum potassium levels (greater than 5.5 mEq per liter).
- Antikaliuretic Therapy or Potassium Supplementation
- Amiloride should not be given to patients receiving other potassium-conserving agents, such as spironolactone or triamterene. Potassium supplementation in the form of medication, potassium-containing salt substitutes or a potassium-rich diet should not be used with amiloride except in severe and/or refractory cases of hypokalemia. Such concomitant therapy can be associated with rapid increases in serum potassium levels. If potassium supplementation is used, careful monitoring of the serum potassium level is necessary.
- Anuria, acute or chronic renal insufficiency, and evidence of diabetic nephropathy are contraindications to the use of amiloride.
- Patients with evidence of renal functional impairment (blood urea nitrogen levels over 30 mg per 100 mL or serum creatinine levels over 1.5 mg per 100 mL) or diabetes mellitus should not receive the drug without careful, frequent and continuing monitoring of serum electrolytes, creatinine, and BUN levels. Potassium retention associated with the use of an antikaliuretic agent is accentuated in the presence of renal impairment and may result in the rapid development of hyperkalemia.
- Amiloride is contraindicated in patients who are hypersensitive to this product.
Warnings
|
HYPERKALEMIA
See full prescribing information for complete Boxed Warning.
* Like other potassium-conserving agents, amiloride may cause hyperkalemia (serum potassium levels greater than 5.5 mEq per liter) which, if uncorrected, is potentially fatal. Hyperkalemia occurs commonly (about 10%) when amiloride is used without a kaliuretic diuretic. This incidence is greater in patients with renal impairment, diabetes mellitus (with or without recognized renal insufficiency), and in the elderly. When amiloride is used concomitantly with a thiazide diuretic in patients without these complications, the risk of hyperkalemia is reduced to about 1-2 percent. It is thus essential to monitor serum potassium levels carefully in any patient receiving amiloride, particularly when it is first introduced, at the time of diuretic dosage adjustments, and during any illness that could affect renal function.
|
Hyperkalemia
- The risk of hyperkalemia may be increased when potassium-conserving agents, including amiloride, are administered concomitantly with an angiotensin-converting enzyme inhibitor, an angiotensin II receptor antagonist, cyclosporine or tacrolimus. Warning signs or symptoms of hyperkalemia include paresthesias, muscular weakness, fatigue, flaccid paralysis of the extremities, bradycardia, shock, and ECG abnormalities. Monitoring of the serum potassium level is essential because mild hyperkalemia is not usually associated with an abnormal ECG.
- When abnormal, the ECG in hyperkalemia is characterized primarily by tall, peaked T waves or elevations from previous tracings. There may also be lowering of the R wave and increased depth of the S wave, widening and even disappearance of the P wave, progressive widening of the QRS complex, prolongation of the PR interval, and ST depression.
Treatment of Hyperkalemia
- If hyperkalemia occurs in patients taking amilordie, the drug should be discontinued immediately.
- If the serum potassium level exceeds 6.5 mEq per liter, active measures should be taken to reduce it. Such measures include the intravenous administration of sodium bicarbonate solution or oral or parenteral glucose with a rapid-acting insulin preparation.
- If needed, a cation exchange resin such as sodium polystyrene sulfonate may be given orally or by enema.
- Patients with persistent hyperkalemia may require dialysis.
Diabetes Mellitus
- In diabetic patients, hyperkalemia has been reported with the use of all potassium-conserving diuretics, including amiloride, even in patients without evidence of diabetic nephropathy. Therefore, amiloride should be avoided, if possible, in diabetic patients and, if it is used, serum electrolytes and renal function must be monitored frequently.
- Amiloride should be discontinued at least three days before glucose tolerance testing.
Metabolic or Respiratory Acidosis
- Antikaliuretic therapy should be instituted only with caution in severely ill patients in whom respiratory acidosis or metabolic acidosis may occur, such as patients with cardiopulmonary disease or poorly controlled diabetes.
- If amiloride is given to these patients, frequent monitoring of acid-base balance is necessary. Shifts in acid-base balance alter the ratio of extracellular/intracellular potassium, and the development of acidosis may be associated with rapid increases in serum potassium levels.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Amiloride in the drug label.
Postmarketing Experience
- Amiloride is usually well tolerated and, except for hyperkalemia (serum potassium levels greater than 5.5 mEq per liter), significant adverse effects have been reported infrequently. Minor adverse reactions were reported relatively frequently (about 20%) but the relationship of many of the reports to amiloride HCl is uncertain and the overall frequency was similar in hydrochlorothiazide treated groups.
- Nausea/anorexia, abdominal pain, flatulence, and mild skin rash have been reported and probably are related to amiloride.
- Other adverse experiences that have been reported with amiloride are generally those known to be associated with diuresis, or with the underlying disease being treated.
- The adverse reactions for amiloride listed in the following table have been arranged into two groups:
- Group 1: Incidence greater than one percent
- Group 2: Incidence one percent or less
- The incidence for group 1 was determined from clinical studies conducted in the United States (837 patients treated with amiloride). The adverse effects listed in group 2 include reports from the same clinical studies and voluntary reports since marketing. The probability of a causal relationship exists between amiloride and these adverse reactions, some of which have been reported only rarely.
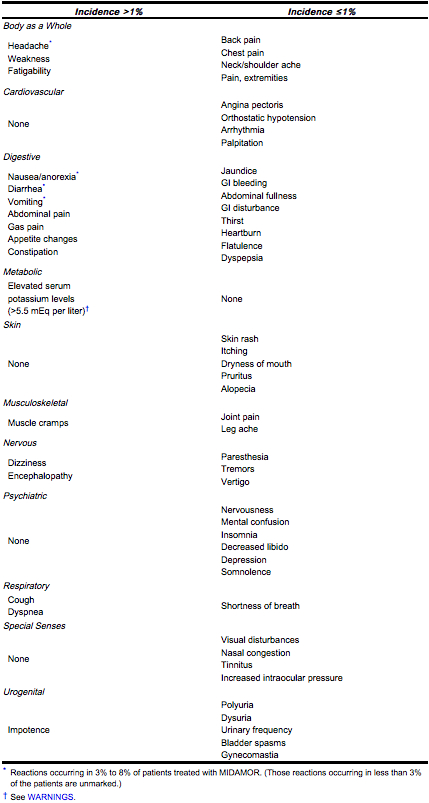
- Causal Relationship Unknown
- Other reactions have been reported but occurred under circumstances where a causal relationship could not be established. However, in these rarely reported events, that possibility cannot be excluded. Therefore, these observations are listed to serve as alerting information to physicians.
- Activation of probable pre-existing peptic ulcer
- Aplastic anemia
- Neutropenia
- Abnormal liver function
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Amiloride in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amiloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amiloride during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Amiloride with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Amiloride with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Amiloride with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Amiloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amiloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amiloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amiloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amiloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amiloride in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Intravenous
Monitoring
Condition1
Description
IV Compatibility
There is limited information regarding IV Compatibility of Amiloride in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- No data are available in regard to overdosage in humans.
- The oral LD50 of amiloride hydrochloride (calculated as the base) is 56 mg/kg in mice and 36 to 85 mg/kg in rats, depending on the strain.
- The most likely signs and symptoms to be expected with overdosage are dehydration and electrolyte imbalance.
Management
- It is not known whether the drug is dialyzable.
- These can be treated by established procedures. Therapy with amiloride should be discontinued and the patient observed closely.
- There is no specific antidote. Emesis should be induced or gastric lavage performed. Treatment is symptomatic and supportive.
- If hyperkalemia occurs, active measures should be taken to reduce the serum potassium levels.
Chronic Overdose
There is limited information regarding Chronic Overdose of Amiloride in the drug label.
Pharmacology
There is limited information regarding Amiloride Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Amiloride Mechanism of Action in the drug label.
Structure
There is limited information regarding Structure of Amiloride in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Amiloride in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Amiloride in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Amiloride in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Amiloride in the drug label.
Condition1
Description
How Supplied
There is limited information regarding Amiloride How Supplied in the drug label.
Storage
There is limited information regarding Amiloride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amiloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amiloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Amiloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Amiloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Midamor®
Look-Alike Drug Names
- aMILoride — amLODIPine[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Viswanathan, V.; Mohan, V.; Subramani, P.; Parthasarathy, N.; Subramaniyam, G.; Manoharan, D.; Sundaramoorthy, C.; Gnudi, L.; Karalliedde, J. (2013). "Effect of spironolactone and amiloride on thiazolidinedione-induced fluid retention in South Indian patients with type 2 diabetes". Clin J Am Soc Nephrol. 8 (2): 225–32. doi:10.2215/CJN.06330612. PMID 23184569. Unknown parameter
|month=ignored (help) - ↑ "http://www.ismp.org". External link in
|title=(help)