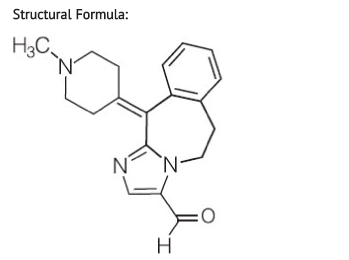
Alcaftadine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Alcaftadine is a antihistamine that is FDA approved for the prophylaxis of allergic conjunctivitis. Common adverse reactions include eye irritation, burning and/or stinging upon instillation, eye redness and eye pruritus, nasopharyngitis, headache, eye discharge, eye swelling, erythema of eyelid, eyelid edema, hypersensitivity, and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- LASTACAFT® is an H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis.
- Instill one drop in each eye once daily. If more than 1 topical ophthalmic medicinal product is being used, each one should be administered at least 5 minutes apart.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alcaftadine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alcaftadine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Alcaftadine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alcaftadine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alcaftadine in pediatric patients.
Contraindications
LASTACAFT® is contraindicated in patients with hypersensitivity to any component in the product.
Warnings
Potential for eye Injury and Contamination
- To minimize eye injury and contamination of the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
Contact Lens Use
- Patients should be advised not to wear a contact lens if their eye is red.
- LASTACAFT® should not be used to treat contact lens-related irritation.
- LASTACAFT® should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
Topical Ophthalmic Use Only
- LASTACAFT® is for topical ophthalmic use only.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Clinical Studies Experience
- The most frequent ocular adverse reactions, occurring in less than 4% of eyes treated with LASTACAFT®, were eye irritation, burning and/or stinging upon instillation, eye redness and eye pruritus.
Non-ocular Adverse Reactions
- The most frequent non-ocular adverse reactions, occurring in less than 3% of subjects with eyes treated with LASTACAFT®, were nasopharyngitis and headache. Some of these events were similar to the underlying disease being studied.
Postmarketing Experience
- The following adverse reactions have been identified during postmarketing use of LASTACAFT® in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions include: eye discharge, eye swelling, erythema of eyelid, eyelid edema, hypersensitivity, and somnolence.
Drug Interactions
There is limited information regarding Alcaftadine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category B. Reproduction studies performed in rats and rabbits revealed no evidence of impaired female reproduction or harm to the fetus due to alcaftadine. Oral doses in rats and rabbits of 20 and 80 mg/kg/day, respectively, produced plasma exposure levels approximately 200 and 9000 times the plasma exposure at the recommended human ocular dose. There are however, no adequate and well controlled studies in pregnant] women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Alcaftadine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Alcaftadine during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LASTACAFT® is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness were observed between elderly and younger subjects.
Gender
There is no FDA guidance on the use of Alcaftadine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Alcaftadine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Alcaftadine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Alcaftadine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Alcaftadine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Alcaftadine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Alcaftadine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Alcaftadine and IV administrations.
Overdosage
There is limited information regarding Alcaftadine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Alcaftadine
| |
| Systematic (IUPAC) name | |
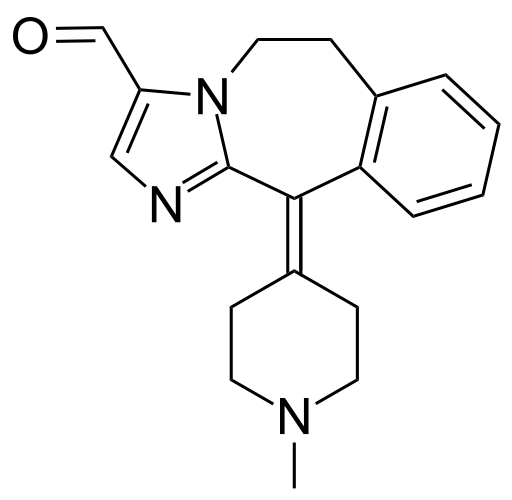
| 2-(1-Methylpiperidin-4-ylidene)-4,7-diazatricyclo[8.4.0.0(3,7)]tetradeca- 1(14),3,5,10,12-pentaene-6-carbaldehyde | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ~2 hrs |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Topical |
Mechanism of Action
- LASTACAFT® is a sterile, topically administered H1 receptor antagonist containing alcaftadine for ophthalmic use.
- Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39.
Contains
- Active: alcaftadine 0.25% (2.5 mg/mL)
- Inactives: benzalkonium chloride 0.005% as a preservative; edetate disodium; sodium phosphate, monobasic; purified water; sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH)
Structure
- LASTACAFT® is a sterile, topically administered H1 receptor antagonist containing alcaftadine for ophthalmic use.
- Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39.
Contains
- Active: alcaftadine 0.25% (2.5 mg/mL)
- Inactives: benzalkonium chloride 0.005% as a preservative; edetate disodium; sodium phosphate, monobasic; purified water; sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH)
Pharmacodynamics
There is limited information regarding Alcaftadine Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Following bilateral topical ocular administration of alcaftadine ophthalmic solution, 0.25%, the mean plasma Cmax of alcaftadine was approximately 60 pg/mL and the median Tmax occurred at 15 minutes. Plasma concentrations of alcaftadine were below the lower limit of quantification (10 pg/mL) by 3 hours after dosing. The mean Cmax of the active carboxylic acid metabolite was approximately 3 ng/mL and occurred at 1 hour after dosing. Plasma concentrations of the carboxylic acid metabolite were below the lower limit of quantification (100 pg/mL) by 12 hours after dosing. There was no indication of systemic accumulation or changes in plasma exposure of alcaftadine or the active metabolite following daily topical ocular administration.
Distribution
- The protein binding of alcaftadine and the active metabolite are 39.2% and 62.7%, respectively.
Metabolism
- The metabolism of alcaftadine is mediated by non-CYP450 cytosolic enzymes to the active carboxylic acid metabolite.
Excretion
- The elimination half-life of the carboxylic acid metabolite is approximately 2 hours following topical ocular administration. Based on data following oral administration of alcaftadine, the carboxylic acid metabolite is primarily eliminated unchanged in the urine.
- In vitro studies showed that neither alcaftadine nor the carboxylic acid metabolite substantially inhibited reactions catalyzed by major CYP450 enzymes.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Alcaftadine was not mutagenic or genotoxic in the Ames test, the mouse lymphoma assay or the mouse micronucleus assay.
- Alcaftadine was found to have no effect on fertility of male and female rats at oral doses up to 20 mg/kg/day (approximately 200 times the plasma exposure at the recommended human ocular dose)
Clinical Studies
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Alcaftadine was not mutagenic or genotoxic in the Ames test, the mouse lymphoma assay or the mouse micronucleus assay.
- Alcaftadine was found to have no effect on fertility of male and female rats at oral doses up to 20 mg/kg/day (approximately 200 times the plasma exposure at the recommended human ocular dose).
How Supplied
- LASTACAFT® (alcaftadine ophthalmic solution) 0.25% is supplied in an opaque, white low-density polyethylene bottle with a white polypropylene cap.
- 3 mL fill in 5 mL bottle NDC 0023-4290-03
Storage
- Store at 15°-25°C (59°-77°F).
Images
Drug Images
{{#ask: Page Name::Alcaftadine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Alcaftadine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Potential for Eye Injury and Sterility of Dropper Tip
- To minimize eye injury and contamination of the dropper tip and solution, patients should be advised to not touch the eyelids or surrounding areas with the dropper tip, as this may contaminate the contents.
Concomitant Use with other Ophthalmic Products or Contact Lenses
- If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
- Patients should be advised not to wear a contact lens if their eye is red. Patients should be advised that LASTACAFT® should not be used to treat contact lens-related irritation. Patients should also be advised to remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
Topical Ophthalmic Use Only
- For topical ophthalmic administration only.
Precautions with Alcohol
Alcohol-Alcaftadine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LASTACAFT ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "LASTACAFT- alcaftadine solution/ drops".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Alcaftadine |Label Name=Alcat 02.jpg
}}
{{#subobject:
|Label Page=Alcaftadine |Label Name=Alcat 03.png
}}
}} }}