Adefovir description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Description
HEPSERA® is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV).
The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy]-phosphinyl]-methoxy]ethyl]adenine. It has a molecular formula of C20H32N5O8P, a molecular weight of 501.48 and the following structural formula:
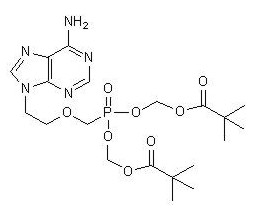 |
Adefovir dipivoxil is a white to off-white crystalline powder with an aqueous solubility of 19 mg/mL at pH 2.0 and 0.4 mg/mL at pH 7.2. It has an octanol/aqueous phosphate buffer (pH 7) partition coefficient (log p) of 1.91.
HEPSERA tablets are for oral administration. Each tablet contains 10 mg of adefovir dipivoxil and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021449s020lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.