Acetyldihydrocodeine: Difference between revisions
Matt Pijoan (talk | contribs) m (Protected "Acetyldihydrocodeine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name | | Verifiedfields = changed | ||
| image | | Watchedfields = changed | ||
| width | | verifiedrevid = 477240369 | ||
| | | IUPAC_name = 3-methoxy-6-acetoxy-(5α,6α)-7,8-Didehydro-4,5-epoxy-17-methylmorphinan | ||
| image = Acetyldihydrocodeine.png | |||
| | | width = 200 | ||
| | | image2 = Acetyldihydrocodeine0.png | ||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|acetyldihydrocodeine}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| pregnancy_AU | | legal_US = Schedule I | ||
| pregnancy_US | |||
<!--Identifiers--> | |||
| legal_AU | | CAS_number_Ref = {{cascite|changed|??}} | ||
| legal_CA | | CAS_number = 3861-72-1 | ||
| legal_UK | | ATC_prefix = R05 | ||
| legal_US | | ATC_suffix = DA12 | ||
| | | PubChem = 5463874 | ||
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| DrugBank = DB01538 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4576412 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = SGY1T84P34 | |||
<!--Chemical data--> | |||
| C=20 | H=25 | N=1 | O=4 | |||
| molecular_weight = 343.417 g/mol | |||
| smiles = O=C(O[C@@H]4[C@@H]5Oc1c2c(ccc1OC)C[C@H]3N(CC[C@]25[C@H]3CC4)C)C | |||
| InChI = 1/C20H25NO4/c1-11(22)24-16-7-5-13-14-10-12-4-6-15(23-3)18-17(12)20(13,19(16)25-18)8-9-21(14)2/h4,6,13-14,16,19H,5,7-10H2,1-3H3/t13-,14+,16-,19-,20-/m0/s1 | |||
| InChIKey = LGGDXXJAGWBUSL-BKRJIHRRBN | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C20H25NO4/c1-11(22)24-16-7-5-13-14-10-12-4-6-15(23-3)18-17(12)20(13,19(16)25-18)8-9-21(14)2/h4,6,13-14,16,19H,5,7-10H2,1-3H3/t13-,14+,16-,19-,20-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = LGGDXXJAGWBUSL-BKRJIHRRSA-N | |||
| synonyms = Acetyldihydrocodeine, Dihydrothebacone, 6-acetyl-7,8-dihydrocodeine | |||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
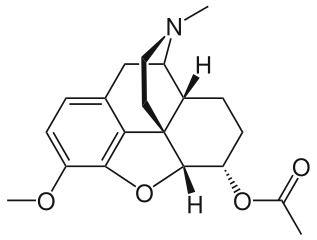
'''Acetyldihydrocodeine''' is an [[opiate]] derivative discovered in Germany in 1914<ref>{{cite journal|author = v. Braun, J.|title = Untersuchungen über Morphium-Alkaloide|journal = Chemische Berichte|year = 1914|volume = 47|issue = 2|pages = 2312–2330|doi = 10.1002/cber.191404702149}}</ref> and was used as a cough suppressant and [[analgesic]]. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of [[Thebacon]], where only the 6-7 [[double bond]] is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of [[dihydrocodeine]] and is metabolised in the liver by demethylation and deacetylation to produce [[dihydromorphine]]. | |||
Since acetyldihydrocodeine has higher [[lipophilicity]] than [[codeine]] and is converted into dihydromorphine rather than [[morphine]], it can be expected to be more potent and longer lasting, and also have higher [[bioavailability]] than codeine. Side effects are similar to those of other [[opiates]] and include [[itching]], [[nausea]] and [[respiratory depression]]. | |||
Although an opioid of low to moderate strength and use in medicine elsewhere in the world, acetyldihydrocodeine is a Schedule I controlled substance in the United States. Its DEA Administrative Controlled Substances Control Number is 9051 and the one salt in use, acetyldihydrocodeine hydrochloride, has a freebase conversion ratio of 0.90. The 2013 annual production quota from the DEA is 2 grammes. | |||
== References == | |||
{{Reflist|2}} | |||
{{Cough and cold preparations}} | {{Cough and cold preparations}} | ||
[[Category: | [[Category:Analgesics]] | ||
[[Category:Morphinans]] | |||
[[Category:Phenol ethers]] | |||
[[Category:Mu-opioid agonists]] | |||
[[Category:Semisynthetic_opioids]] | |||
[[Category:Drug]] | |||
Latest revision as of 17:22, 9 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | Acetyldihydrocodeine, Dihydrothebacone, 6-acetyl-7,8-dihydrocodeine |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H25NO4 |
| Molar mass | 343.417 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Acetyldihydrocodeine is an opiate derivative discovered in Germany in 1914[1] and was used as a cough suppressant and analgesic. It is not commonly used, but has activity similar to other opiates. Acetyldihydrocodeine is a very close relative derivative of Thebacon, where only the 6-7 double bond is unsaturated. Acetyldihydrocodeine can be described as the 6-acetyl derivative of dihydrocodeine and is metabolised in the liver by demethylation and deacetylation to produce dihydromorphine.
Since acetyldihydrocodeine has higher lipophilicity than codeine and is converted into dihydromorphine rather than morphine, it can be expected to be more potent and longer lasting, and also have higher bioavailability than codeine. Side effects are similar to those of other opiates and include itching, nausea and respiratory depression.
Although an opioid of low to moderate strength and use in medicine elsewhere in the world, acetyldihydrocodeine is a Schedule I controlled substance in the United States. Its DEA Administrative Controlled Substances Control Number is 9051 and the one salt in use, acetyldihydrocodeine hydrochloride, has a freebase conversion ratio of 0.90. The 2013 annual production quota from the DEA is 2 grammes.
References
- ↑ v. Braun, J. (1914). "Untersuchungen über Morphium-Alkaloide". Chemische Berichte. 47 (2): 2312–2330. doi:10.1002/cber.191404702149.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Analgesics
- Morphinans
- Phenol ethers
- Mu-opioid agonists
- Semisynthetic opioids
- Drug