Acetylcysteine (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetylcysteine (injection) is an antioxidant, respiratory system agent that is FDA approved for the treatment of acetaminophen overdose, adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions in such conditions as chronic bronchopulmonary disease (chronic emphysema, emphysema with bronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis and primary amyloidosis of the lung), acute bronchopulmonary disease (pneumonia, bronchitis, tracheobronchitis), pulmonary complications of cystic fibrosis, tracheostomy care, pulmonary complications associated with surgery, use during anesthesia, post-traumatic chest conditions, atelectasis due to mucous obstruction, diagnostic bronchial studies (bronchograms, bronchospirometry, and bronchial wedge catheterization). Common adverse reactions include rash, urticaria/facial flushing and pruritus, diarrhea, nausea, vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acetaminophen overdose
- Acetylcysteine Injection is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen. Overdose incidences are divided into two types; Acute Ingestion or Repeated Supratherapeutic Ingestion (RSI).
- On admission for suspected acetaminophen overdose, a serum blood sample should be drawn at least 4 hours after ingestion to determine the acetaminophen level and will serve as a basis for determining the need for treatment with acetylcysteine. If the patient presents after 4 hours post-ingestion, the serum acetaminophen sample should be determined immediately.
- Acetylcysteine Injection should be administered within 8 hours from acetaminophen ingestion for maximal protection against hepatic injury for patients whose serum acetaminophen levels fall above the "possible" toxicity line on the Rumack-Matthew nomogram (line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours); [see Acetaminophen Assays – Interpretation and Methodology]. If the time of ingestion is unknown, or the serum acetaminophen level is not available, cannot be interpreted, or is not available within the 8 hour time interval from acetaminophen ingestion, Acetylcysteine Injection should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen, regardless of the quantity reported to have been ingested.
- The aspartate aminotransferase (AST, SGOT), alanine aminotranferase (ALT, SGPT), bilirubin, prothrombin time, creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes also should be determined in order to monitor hepatic and renal function and electrolyte and fluid balance.
- NOTE: The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
Acetaminophen Assays Interpretation and Methodology – Acute Ingestion
- The acute ingestion of acetaminophen in quantities of 150 mg/kg or greater may result in hepatic toxicity. However, the reported history of the quantity of a drug ingested as an overdose is often inaccurate and is not a reliable guide to therapy of the overdose. Therefore, plasma or serum acetaminophen concentrations, determined as early as possible, but no sooner than four hours following an acute overdose, are essential in assessing the potential risk of hepatotoxicity. If an assay for acetaminophen cannot be obtained, it is necessary to assume that the overdose is potentially toxic.
Interpretation of Acetaminophen Assays
- When results of the plasma acetaminophen assay are available, refer to the nomogram in Figure 1 to determine if plasma concentration is in the potentially toxic range. Values above the line connecting 200 mcg/mL at 4 hours with 50 mcg/mL at 12 hours (probable line) are associated with a probability of hepatic toxicity if an antidote is not administered.
- If the predetoxification plasma level is above the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), continue with maintenance doses of acetylcysteine. It is better to err on the safe side and thus this line, defining possible toxicity, is plotted 25% below the line defining probable toxicity.
- If the predetoxification plasma level is below the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), there is minimal risk of hepatic toxicity, and Acetylcysteine treatment may be discontinued.
- Estimating Potential for Hepatotoxicity: The following depiction of the Rumack- Matthew nomogram has been developed to estimate the probability that plasma levels in relation to intervals post-ingestion will result in hepatotoxicity.
- The Rumack-Matthew nomogram may underestimate the risk for hepatotoxicity in some patients with risk factors such as chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid).
Figure 1. Rumack-Matthew Nomogram:
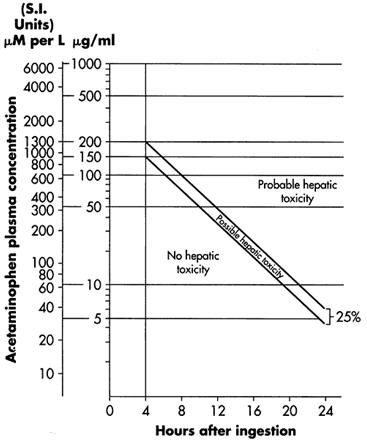
- Figure 1. Michael J Hodgman, Alexander R Garrard, A Review of Acetaminophen Poisoning. Crit Care Clin. 28 (2012) 499-516. Stephen J. Wolf, Kennon Heard, et.al, Clinical Policy: Critical Issues in the Management of Patients Presenting to the Emergency Department with Acetaminophen Overdose. Ann Emerg Med. 2007:50:292-313.
Acetaminophen Assays Interpretation and Methodology – Repeated Supratherapeutic Ingestion
- Repeated Supratherapeutic Ingestion (RSI) is defined as ingestion of acetaminophen at doses higher than those recommended for extended periods of time. The nomogram does not apply to patients with RSI. Treatment is based on the acetaminophen and elevated AST/ALT levels indicative of potential toxicity due to acetaminophen. For specific treatment information regarding the clinical management of repeated supratherapeutic acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
Figure 2. Acetylcysteine Injection Treatment Flow Chart
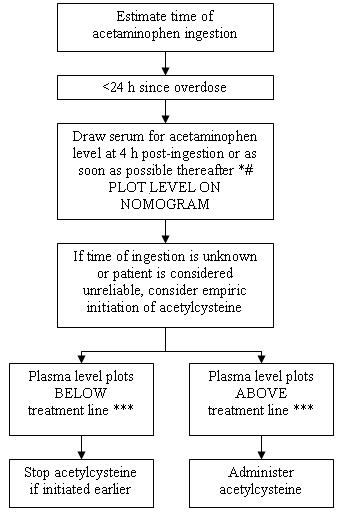
- 1Acetaminophen levels drawn less than 4 hours post-ingestion may be misleading.
- 2With an extended-release preparation, an acetaminophen level drawn less than 8 hours post-ingestion may be misleading. Draw a second level at 4 to 6 hours after the initial level. If either falls above the toxicity line, acetylcysteine treatment should be initiated.
- 3Acetylcysteine may be withheld until acetaminophen assay results are available as long as initiation of treatment is not delayed beyond 8 hours post-ingestion. If more than 8 hours post-ingestion, start acetylcysteine treatment immediately.
Dosing Information
- The total dose of Acetylcysteine Injection is 300 mg/kg given as 3 separate doses and administered over a total of 21 hours. Please refer to the guidelines below for dose preparation based upon patient weight. The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction (see Tables 1 and 2).
Administration Instructions (Three-Bag Method: Loading, Second and Third Dose)
- Dosing for Patients who weigh 5 kg to 20 kg (Table 1):
- Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent* administered over 16 hrs
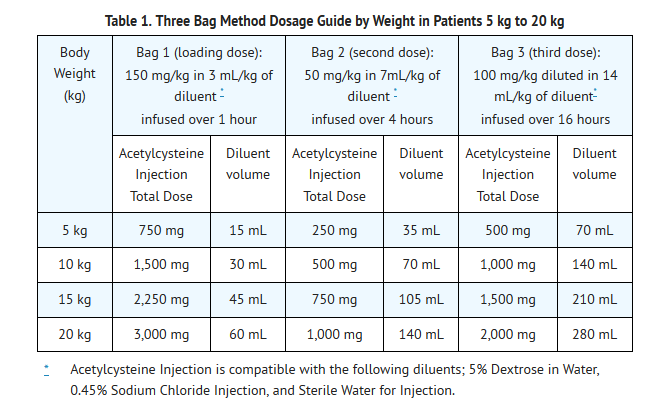
- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- Dosing for patients who weigh 21 kg to 40 kg (Table 2):
- Loading Dose: 150 mg/kg diluted in 100 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 250 mL of diluent* administered over 4 hrs
- Third Dose: : 100 mg/kg diluted in 500 mL of diluent* administered over 16 hrs
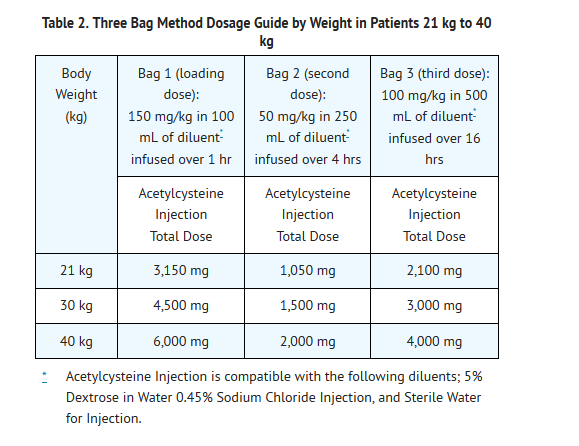
- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction.
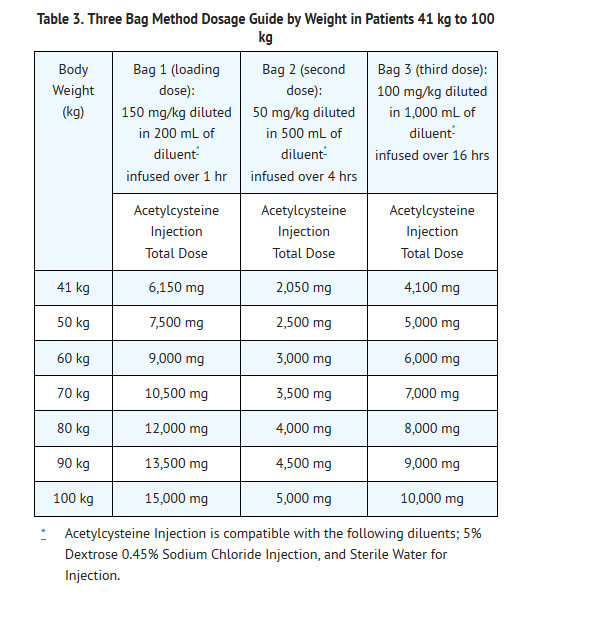
- Dosing for patients who weigh 41 kg to 100 kg (Table 3):
- Loading Dose: 150 mg/kg diluted in 200 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 500 mL of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 1,000 mL of diluent administered over 16 hrs
Patients Weighing More Than 100 kg
- No specific studies have been conducted to evaluate the use of or necessity of dosing adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. The dose of Acetylcysteine Injection recommended in these patients should be a loading dose of 15,000 mg infused over a period of one hour followed by a first maintenance dose of 5,000 mg over 4 hours and a second maintenance dose of 10,000 mg over 16 hours (Table 3).
Continued Therapy beyond 21 Hours
- While there is no clinical trial data to support infusions beyond 21 hours there is literature that supports continued infusion of acetylcysteine in some rare instances. In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease, the absorption and/or the half-life of acetaminophen may be prolonged, in such cases consideration should be given to the need for continued infusion of N-acetylcysteine beyond 21 hours. Acetaminophen levels and ALT/AST & INR should be checked before the end of the 21-hour infusion. If acetaminophen levels are still detectable, or in cases in which the ALT/AST are still increasing or the INR remains elevated, the infusion should be continued, and the treating physician should contact a US regional poison center at 1-800-222-1222, or alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations.
Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as clinically needed. If the volume of the infusion is not adjusted, fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
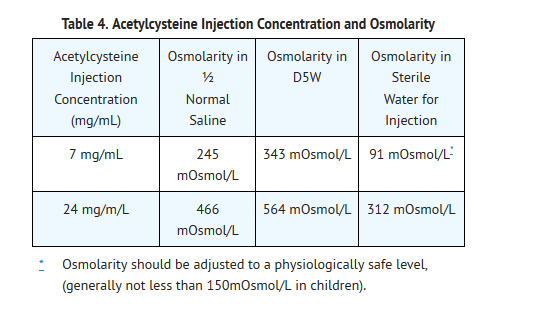
- As Acetylcysteine Injection is hyperosmolar (2600 mOsmol/L), caution is advised when the diluent volume is decreased as the hyperosmolarity of the solution is increased. See Table 4 below for examples.
- Single dose vial, preservative-free, discard unused portion. If vial was previously opened, do not use for intravenous administration.
- Stability studies indicate that the diluted solution is stable for 24 hours at controlled room temperature.
- Note: The color of Acetylcysteine Injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product.
Renal Impairment
- No data are available to determine if a dose adjustment in patients with moderate or severe renal impairment is required.
Hepatic Impairment
- Although there was a threefold increase in acetylcysteine plasma concentrations in patients with hepatic cirrhosis, no data are available to determine if a dose adjustment in these patients is required. The published medical literature does not indicate that the dose of acetylcysteine in patients with hepatic impairment should be reduced.
DOSAGE FORMS & STRENGTHS
- Each single dose vial contains 6g/30mL (200 mg/mL) of Acetylcysteine. Acetylcysteine Injection is sterile and can be used for intravenous administration.
Administration of anesthesia for procedure
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Atelectasis, Due to mucous obstruction
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Bronchopulmonary disease, acute
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Complication of surgical procedure - Respiratory complication
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Cystic fibrosis, Pulmonary complications; Adjunct
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Diagnostic procedure on lower respiratory tract
- Dosing Information
- Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally
Disease of respiratory system, chronic
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Disease of respiratory system, chronic: direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Disease of respiratory system, chronic: percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Disease of respiratory system, chronic: intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Tracheostomy care
- Dosing Information
- Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (injection) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acetaminophen overdose
- Dosing Information
- Oral: initial, 140 mg/kg ORALLY, then 70 mg/kg every 4 hours for 17 doses starting 4 hours after loading dose
- IV: body weight 5 to 20 kg: loading dose, 150 mg/kg in 3 mL/kg of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 7 mL/kg of compatible solution IV over 4 hours, followed by 100 mg/kg in 14 mL/kg of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary
- IV: body weight 21 kg to 40 kg: loading dose, 150 mg/kg in 100 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 250 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 500 mL of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients less than 40 kg or requiring fluid restriction as clinically necessary
- IV: body weight 41 to 100 kg, loading dose, 150 mg/kg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 500 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary [3]
- IV: body weight over 100 kg, loading dose, 15,000 mg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 5000 mg in 500 mL of compatible solution IV over 4 hours, followed by 10,000 mg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary
Administration of anesthesia for procedure
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Atelectasis, Due to mucous obstruction
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Bronchopulmonary disease, acute
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Complication of surgical procedure - Respiratory complication
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Cystic fibrosis, Pulmonary complications; Adjunct
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Diagnostic procedure on lower respiratory tract
- Dosing Information
- Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally
Disease of respiratory system, chronic
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Tracheostomy care
- Dosing Information
- Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (injection) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (injection) in pediatric patients.
Contraindications
- Acetylcysteine Injection is contraindicated in patients with previous anaphylactoid reactions to acetylcysteine.
Warnings
Anaphylactoid Reactions
- Serious anaphylactoid reactions, including death in a patient with asthma, have been reported in patients administered acetylcysteine intravenously.
- Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. Anaphylactoid reactions (defined as the occurrence of an acute hypersensitivity reaction during acetylcysteine administration including rash, hypotension, wheezing, and/or shortness of breath) have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion.If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as an anaphylactoid reaction. This usually entails administering antihistaminic drugs and in severe cases may require administration of epinephrine. In addition, the acetylcysteine infusion may be interrupted until treatment of the anaphylactoid symptoms has been initiated and then carefully restarted. If the anaphylactoid reaction returns upon reinitiation of treatment or increases in severity, intravenous acetylcysteine should be discontinued and alternative patient management should be considered.
Monitoring patients with asthma
- Acetylcysteine Injection should be used with caution in patients with asthma, or where there is a history of bronchospasm.
Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as needed. If volume is not adjusted fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
- For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
- After proper administration of acetylcysteine, an increased volume of liquified bronchial secretions may occur. When cough is inadequate, the open airway must be maintained by mechanical suction if necessary. When there is a mechanical block due to foreign body or local accumulation, the airway should be cleared by endotracheal aspiration, with or without bronchoscopy. Asthmatics under treatment with acetylcysteine should be watched carefully. Most patients with bronchospasm are quickly relieved by the use of a bronchodilator given by nebulization. If bronchospasm progresses, this medication should be discontinued immediately.
Adverse Reactions
Clinical Trials Experience
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In the literature the most frequently reported adverse reactions attributed to intravenous acetylcysteine administration were rash, urticaria and pruritus. The frequency of adverse reactions has been reported to be between 0.2% and 20.8%, and they most commonly occur during the initial loading dose of acetylcysteine.
Loading Dose/Infusion Rate Study
- The incidence of drug-related adverse reactions occurring within the first 2 hours following acetylcysteine administration reported in a randomized study in patients with acetaminophen poisoning is presented in Table 5 by preferred term. In this study patients were randomized to a 15-minute or a 60-minute loading dose regimen.
- Within the first 2 hours following intravenous acetylcysteine administration, 17% developed an anaphylactoid reaction (18% in the 15-minute treatment group; 14% in the 60-minute treatment group) in this randomized, open-label, multi-center clinical study conducted in Australia to compare the rates of anaphylactoid reactions between two rates of infusion for the intravenous acetylcysteine loading dose [ see WARNINGS (Section 5) and CLINACAL STUDIES - Loading Dose/InfusionRate Study (Section 14)

Postmarketing Safety Study
- A large multi-center study was performed in Canada where data were collected from patients who were treated with intravenous acetylcysteine for acetaminophen overdose between 1980 and 2005. This study evaluated 4709 adult cases and 1905 pediatric cases. The incidence of anaphylactoid reactions in adult (overall incidence 7.9%) and pediatric (overall incidence 9.5%) patients is presented in Tables 6 and 7.


Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Acetylcysteine (injection) in the drug label.
Drug Interactions
- No drug-drug interaction studies have been conducted.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category B
- There are no adequate and well-controlled studies of Acetylcysteine Injection in pregnant women. However, limited case reports of pregnant women exposed to acetylcysteine during various trimesters did not report any adverse maternal, fetal or neonatal outcomes.
- There are published reports on four pregnant women with acetaminophen toxicity, who were treated with oral or intravenous acetylcysteine at the time of delivery. Acetylcysteine crossed the placenta and was measurable following delivery in serum and cord blood of three viable infants and in cardiac blood of a fourth infant at autopsy (22 weeks gestational age who died 3 hours after birth). No adverse sequelae developed in the three viable infants. All mothers recovered and none of the infants had evidence of acetaminophen poisoning.
- Reproduction studies were performed in rats at oral doses up to 2,000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison) and in rabbits at oral doses up to 1,000 mg/kg/day (1.1 times the recommended total human intravenous dose of 300 mg/kg based on body surface area comparison). No effects on fertility or harm to the fetus due to acetylcysteine were observed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetylcysteine (injection) in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Acetylcysteine (injection) during labor and delivery.
Nursing Mothers
- It is not known whether Acetylcysteine Injection is present in human milk. Because many drugs are excreted in human milk, caution should be exercised when acetylcysteine is administered to a nursing woman. Based on the pharmacokinetics of acetylcysteine, it should be nearly completely cleared 30 hours after administration. Nursing women may consider resuming nursing 30 hours after administration.
Pediatric Use
- No adverse effects were noted during intravenous infusion with acetylcysteine at a mean rate of 4.2 mg/kg/h for 24 hours to 10 preterm newborns ranging in gestational age from 25 to 31 weeks and in weight from 500 to 1380 grams in one study or in 6 newborns ranging in gestational age from 26 to 30 weeks and in weight from 520 to 1335 grams infused with acetylcysteine at 0.1 to 1.3 mg/kg/h for 6 days. Elimination of acetylcysteine was slower in these infants than in adults; mean elimination half-life was 11 hours. There are no adequate and well-controlled studies in pediatric patients.
Geriatic Use
- The clinical studies do not provide a sufficient number of geriatric subjects to determine whether the elderly respond differently.
Gender
- There is no FDA guidance on the use of Acetylcysteine (injection) with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Acetylcysteine (injection) with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Acetylcysteine (injection) in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Acetylcysteine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Acetylcysteine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Acetylcysteine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- The total dose of Acetylcysteine Injection is 300 mg/kg given as 3 separate doses and administered over a total of 21 hours. Please refer to the guidelines below for dose preparation based upon patient weight. The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction (see Tables 1 and 2).
Administration Instructions (Three-Bag Method: Loading, Second and Third Dose)
- Dosing for Patients who weigh 5 kg to 20 kg (Table 1):
- Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent* administered over 16 hrs

- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- Dosing for patients who weigh 21 kg to 40 kg (Table 2):
- Loading Dose: 150 mg/kg diluted in 100 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 250 mL of diluent* administered over 4 hrs
- Third Dose: : 100 mg/kg diluted in 500 mL of diluent* administered over 16 hrs

- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction.
- Dosing for patients who weigh 41 kg to 100 kg (Table 3):
- Loading Dose: 150 mg/kg diluted in 200 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 500 mL of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 1,000 mL of diluent administered over 16 hrs

Patients Weighing More Than 100 kg
- No specific studies have been conducted to evaluate the use of or necessity of dosing adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. The dose of Acetylcysteine Injection recommended in these patients should be a loading dose of 15,000 mg infused over a period of one hour followed by a first maintenance dose of 5,000 mg over 4 hours and a second maintenance dose of 10,000 mg over 16 hours (Table 3).
Continued Therapy beyond 21 Hours
- While there is no clinical trial data to support infusions beyond 21 hours there is literature that supports continued infusion of acetylcysteine in some rare instances. In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease, the absorption and/or the half-life of acetaminophen may be prolonged, in such cases consideration should be given to the need for continued infusion of N-acetylcysteine beyond 21 hours. Acetaminophen levels and ALT/AST & INR should be checked before the end of the 21-hour infusion. If acetaminophen levels are still detectable, or in cases in which the ALT/AST are still increasing or the INR remains elevated, the infusion should be continued, and the treating physician should contact a US regional poison center at 1-800-222-1222, or alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations.
Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as clinically needed. If the volume of the infusion is not adjusted, fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
- As Acetylcysteine Injection is hyperosmolar (2600 mOsmol/L), caution is advised when the diluent volume is decreased as the hyperosmolarity of the solution is increased. See Table 4 below for examples.

- Single dose vial, preservative-free, discard unused portion. If vial was previously opened, do not use for intravenous administration.
- Stability studies indicate that the diluted solution is stable for 24 hours at controlled room temperature.
- Note: The color of Acetylcysteine Injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product.
Renal Impairment
- No data are available to determine if a dose adjustment in patients with moderate or severe renal impairment is required.
Hepatic Impairment
- Although there was a threefold increase in acetylcysteine plasma concentrations in patients with hepatic cirrhosis, no data are available to determine if a dose adjustment in these patients is required. The published medical literature does not indicate that the dose of acetylcysteine in patients with hepatic impairment should be reduced.
DOSAGE FORMS & STRENGTHS
- Each single dose vial contains 6g/30mL (200 mg/mL) of Acetylcysteine. Acetylcysteine Injection is sterile and can be used for intravenous administration.
Monitoring
- There is limited information regarding Monitoring of Acetylcysteine (injection) in the drug label.
- Description
IV Compatibility
- There is limited information regarding IV Compatibility of Acetylcysteine (injection) in the drug label.
Overdosage
- Single intravenous doses of acetylcysteine at 1000 mg/kg in mice, 2445 mg/kg in rats, 1500 mg/kg in guinea pigs, 1200 mg/kg in rabbits and 500 mg/kg in dogs were lethal. Symptoms of acute toxicity were ataxia, hypoactivity, labored respiration, cyanosis, loss of righting reflex and convulsions.
Pharmacology
There is limited information regarding Acetylcysteine (injection) Pharmacology in the drug label.
Mechanism of Action
Acetaminophen Overdose:
- Acetaminophen is absorbed from the upper gastrointestinal tract with peak plasma levels occurring between 30 and 60 minutes after therapeutic doses and usually within 4 hours following an overdose. It is extensively metabolized in the liver to form principally the sulfate and glucoronide conjugates which are excreted in the urine. A small fraction of an ingested dose is metabolized in the liver by isozyme CYP2E1 of the cytochrome P-450 mixed function oxidase enzyme system to form a reactive, potentially toxic, intermediate metabolite. The toxic metabolite preferentially conjugates with hepatic glutathione to form nontoxic cysteine and mercapturic acid derivatives, which are then excreted by the kidney. Recommended therapeutic doses of acetaminophen are not believed to saturate the glucuronide and sulfate conjugation pathways and therefore are not expected to result in the formation of sufficient reactive metabolite to deplete glutathione stores. However, following ingestion of a large overdose, the glucuronide and sulfate conjugation pathways are saturated resulting in a larger fraction of the drug being metabolized via the cytochrome P-450 pathway and therefore, the amount of acetaminophen metabolized to the reactive intermediate increases. The increased formation of the reactive metabolite may deplete the hepatic stores of glutathione with subsequent binding of the metabolite to protein molecules within the hepatocyte resulting in cellular necrosis.
Acetylcysteine Intravenous Treatment:
- Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. It is most effective when given early, with benefit seen principally in patients treated within 8-10 hours of the overdose. Acetylcysteine likely protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite.
Structure
- Acetylcysteine injection is an intravenous antidote for the treatment of acetaminophen overdose. Acetylcysteine is the nonproprietary name for the N-acetyl derivative of the naturally occurring amino acid, L-cysteine (N-acetyl-L-cysteine, NAC). The compound is a white crystalline powder, which melts in the range of 104° to 110°C and has a very slight odor. The molecular formula of the compound is C5H9NO3S, and its molecular weight is 163.2. Acetylcysteine has the following structural formula:

- Acetylcysteine Injection is supplied as a sterile solution in vials containing 20% w/v (200 mg/mL) acetylcysteine. The pH of the solution ranges from 6.0 to 7.5. Acetylcysteine Injection contains the following inactive ingredients: 0.5 mg/mL disodium edetate, sodium hydroxide (used for pH adjustment), and water for injection, USP.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Acetylcysteine (injection) in the drug label.
Pharmacokinetics
Distribution:
- The steady-state volume of distribution (Vdss) and the protein binding for acetylcysteine were reported to be 0.47 liter/kg and 83%, respectively.
Metabolism:
- Acetylcysteine may form cysteine, disulfides and conjugates in vivo (N, N'-diacetylcysteine, N-acetylcysteine-cysteine, N-acetylcysteine- glutathione, N-acetylcysteine-protein, etc). Based on published data, it was reported that after an oral dose of 35S-acetylcysteine, about 22% of total radioactivity was excreted in urine after 24 hours. No metabolites were identified.
Elimination:
- After a single intravenous dose of acetylcysteine, the plasma concentration of total acetylcysteine declined in a poly-exponential decay manner with a mean terminal half-life (T1/2) of 5.6 hours. The mean clearance (CL) for acetylcysteine was reported to be 0.11 liter/hr/kg and renal CL constituted about 30% of total CL.
Special Populations:
- Gender: Adequate information is not available to assess if there are differences in pharmacokinetics (PK) between males and females.
- Pediatric: The mean elimination T1/2 of acetylcysteine is longer in newborns (11 hours) than in adults (5.6 hours). Pharmacokinetic information is not available in other age groups.
- Pregnant Women: In four pregnant women with acetaminophen toxicity, oral or I.V. acetylcysteine was administered at the time of delivery. Acetylcysteine was detected in the cord blood of 3 viable infants and in cardiac blood of a fourth infant sampled at autopsy [see Pregnancy (8.1)].
- Hepatic Impairment: In subjects with severe liver damage, i.e., cirrhosis due to alcohol (with Child-Pugh score of 7 to 13), or primary and/or secondary biliary cirrhosis (with Child-Pugh score of 5 to 7), mean T1/2 increased by 80% while mean CL decreased by 30% compared to the control group.
- Renal Impairment: Pharmacokinetic information is not available in patients with renal impairment.
- Geriatric Patients: Adequate information on acetylcysteine PK in geriatric patients is not available.
Nonclinical Toxicology
Carcinogenesis & Mutageneis & Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of acetylcysteine.
- Acetylcysteine was not genotoxic in the Ames test or the in vivo mouse micronucleus test. It was, however, positive in the in vitro mouse lymphoma cell (L5178Y/TK+/-) forward mutation test.
- Treatment of male rats with acetylcysteine at an oral dose of 250 mg/kg/day for 15 weeks (0.1 times the recommended human dose of 300 mg/kg) did not affect the fertility or general reproductive performance.
Clinical Studies
Loading Dose/Infusion Rate Study
- A randomized, open-label, multi-center clinical study was conducted in Australia to compare the rates of anaphylactoid reactions between two rates of infusion for the intravenous acetylcysteine loading dose. One hundred nine subjects were randomized to a 15 minute infusion rate and seventy-one subjects were randomized to a 60 minute infusion rate. The loading dose was 150 mg/kg followed by a maintenance dose of 50 mg/kg over 4 hours and then 100 mg/kg over 16 hours. Of the 180 patients, 27% were male and 73% were female. Ages ranged from 15 to 83 years, with the mean age being 29.9 years (±13.0).
- A subgroup of 58 subjects (33 in the 15-minute treatment group; 25 in the 60-minute treatment group) was treated within 8 hours of acetaminophen ingestion. No hepatotoxicity occurred within this subgroup; however with 95% confidence, the true hepatotoxicity rates could range from 0% to 9% for the 15-minute treatment group and from 0% to 12% for the 60-minute treatment group.
Observational Study
- An open-label, observational database contained information on 1,749 patients who sought treatment for acetaminophen overdose over a 16-year period. Of the 1,749 patients, 65% were female, 34% were male and less than 1% was transgender. Ages ranged from 2 months to 96 years, with 71.4% of the patients falling in the 16 to 40 year old age bracket. A total of 399 patients received acetylcysteine treatment. A post-hoc analysis identified 56 patients who (1) were at high or probable risk for hepatotoxicity (APAP greater than 150 mg/L at the four hours line according to the Australian nomogram) and (2) had a liver function test. Of the 53 patients who were treated with intravenous acetylcysteine (300 mg/kg intravenous acetylcysteine administered over 20 to 21 hours) within 8 hours, two (4%) developed hepatotoxicity (AST or ALT greater than 1,000 U/L). Twenty-one of 48 (44%) patients treated with acetylcysteine after 15 hours developed hepatotoxicity. The actual number of hepatotoxicity outcomes may be higher than what is reported here. For patients with multiple admissions for acetaminophen overdose, only the first overdose treated with intravenous acetylcysteine was examined. Hepatotoxicity may have occurred in subsequent admissions.
- Evaluable data were available from a total of 148 pediatric patients (less than 16 years of age) who were admitted for poisoning following ingestion of acetaminophen, of whom 23 were treated with intravenous acetylcysteine. Of the 23 patients who received intravenous acetylcysteine treatment, 3 patients (13%) had an adverse reaction (anaphylactoid reaction, rash and flushing, transient erythema). There were no deaths of pediatric patients. None of the pediatric patients receiving intravenous acetylcysteine developed hepatotoxicity while two patients not receiving intravenous acetylcysteine developed hepatotoxicity. The number of pediatric patients is too small to provide a statistically significant finding of efficacy, however the results appear to be consistent to those observed for adults.
- Postmarketing Safety Study [see 6.1 Clinical Studies Experience]
How Supplied
- Acetylcysteine Injection is available as a 20% solution (200 mg/mL) in 30 mL single dose glass vials. Each single dose vial contains 6 g/30 mL (200 mg/mL) of Acetylcysteine. Acetylcysteine Injection is sterile and can be used for intravenous administration.

- Do not use previously opened vials for intravenous administration.
- Note: The color of Acetylcysteine Injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product.
- The stopper in the Acetylcysteine Injection vial is formulated with a synthetic base-polymer and does not contain Natural Rubber Latex, Dry Natural Rubber, or blends of Natural Rubber.
Storage
Storage
- Store unopened vials at controlled room temperature, 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- To report SUSPECTED ADVERSE REACTIONS, contact West-Ward Pharmaceutical Corp. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- For Product Inquiry call 1-877-845-0689.
Images
Drug Images
{{#ask: Page Name::Acetylcysteine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Acetylcysteine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Sensitivity to acetylcysteine: Patients should be advised to report to their physician any history of sensitivity to acetylcysteine.
- Asthma: Patients should be advised to report to their physician any history of asthma
- For all questions concerning adverse reactions associated with the use of this product or for Inquiries concerning our products, please contact us at 1-800-551-7176.
- For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
Precautions with Alcohol
- Alcohol-Acetylcysteine (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Acetadote, Acys-5, Mucomyst.
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Acetylcysteine (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Acetylcysteine (injection) |Label Name=Acetylcysteine (injection)11.png
}}
{{#subobject:
|Label Page=Acetylcysteine (injection) |Label Name=Acetylcysteine (injection)11.png
}}