Abacavir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF HYPERSENSITIVITY REACTIONS, LACTIC ACIDOSIS, AND SEVERE HEPATOMEGALY
See full prescribing information for complete Boxed Warning.
* Hypersensitivity Reactions: Serious and sometimes fatal hypersensitivity reactions have been associated with ZIAGEN® (abacavir sulfate).
|
Overview
Abacavir is a nucleoside analogue that is FDA approved for the treatment of HIV-1 infection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, headache, malaise and fatigue, nausea and vomiting, and dreams/sleep disorders..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- ZIAGEN Tablets and Oral Solution, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection.
Additional important information on the use of ZIAGEN for treatment of HIV-1 infection:
ZIAGEN is one of multiple products containing abacavir. Before starting ZIAGEN, review medical history for prior exposure to any abacavir-containing product in order to avoid reintroduction in a patient with a history of hypersensitivity to abacavir
Dosage
A Medication Guide and Warning Card that provide information about recognition of hypersensitivity reactions should be dispensed with each new prescription and refill. ZIAGEN may be taken with or without food.
Adult Patients
The recommended oral dose of ZIAGEN for adults is 600 mg daily, administered as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents.
Patients With Hepatic Impairment
The recommended dose of ZIAGEN in patients with mild hepatic impairment (Child-Pugh score 5 to 6) is 200 mg twice daily. To enable dose reduction, ZIAGEN Oral Solution (10 mL twice daily) should be used for the treatment of these patients. The safety, efficacy, and pharmacokinetic properties of abacavir have not been established in patients with moderate to severe hepatic impairment; therefore, ZIAGEN is contraindicated in these patients.
3 DOSAGE FORMS AND STRENGTHS
ZIAGEN Tablets contain 300 mg of abacavir as abacavir sulfate. The tablets are yellow, biconvex, scored, capsule-shaped, film-coated, and imprinted with “GX 623” on both sides.
ZIAGEN Oral Solution contains 20 mg/mL of abacavir as abacavir sulfate. The solution is a clear to opalescent, yellowish, strawberry-banana-flavored liquid.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
Pediatric Patients
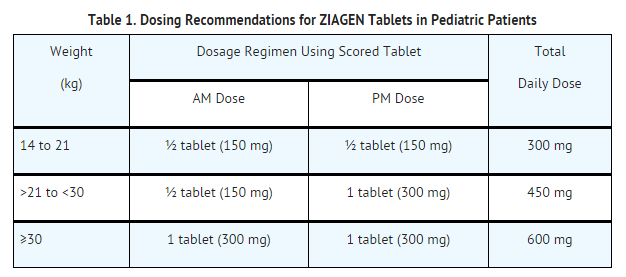
The recommended oral dose of ZIAGEN Oral Solution in HIV-1-infected pediatric patients aged 3 months and older is 8 mg/kg twice daily (up to a maximum of 300 mg twice daily) in combination with other antiretroviral agents.
ZIAGEN is also available as a scored tablet for HIV-1-infected pediatric patients weighing greater than or equal to 14 kg for whom a solid dosage form is appropriate. Before prescribing ZIAGEN Tablets, children should be assessed for the ability to swallow tablets. If a child is unable to reliably swallow ZIAGEN Tablets, the oral solution formulation should be prescribed. The recommended oral dosage of ZIAGEN Tablets for HIV-1-infected pediatric patients is presented in Table 1.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
ZIAGEN is contraindicated in patients with:
previously demonstrated hypersensitivity to abacavir or any other component of the products. NEVER restart ZIAGEN or any other abacavir-containing product following a hypersensitivity reaction to abacavir, regardless of HLA-B*5701 status [see Warnings and Precautions (5.1), Adverse Reactions (6)]. moderate or severe hepatic impairment
Warnings
|
WARNING: RISK OF HYPERSENSITIVITY REACTIONS, LACTIC ACIDOSIS, AND SEVERE HEPATOMEGALY
See full prescribing information for complete Boxed Warning.
* Hypersensitivity Reactions: Serious and sometimes fatal hypersensitivity reactions have been associated with ZIAGEN® (abacavir sulfate).
|
Hypersensitivity Reaction
Serious and sometimes fatal hypersensitivity reactions have been associated with ZIAGEN and other abacavir-containing products. Patients who carry the HLA-B*5701 allele are at high risk for experiencing a hypersensitivity reaction to abacavir. Prior to initiating therapy with abacavir, screening for the HLA-B*5701 allele is recommended; this approach has been found to decrease the risk of a hypersensitivity reaction. Screening is also recommended prior to reinitiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir. For HLA-B*5701-positive patients, treatment with an abacavir-containing regimen is not recommended and should be considered only with close medical supervision and under exceptional circumstances when the potential benefit outweighs the risk. HLA-B*5701-negative patients may develop a hypersensitivity reaction to abacavir; however, this occurs significantly less frequently than in HLA-B*5701-positive patients. Regardless of HLA-B*5701 status, permanently discontinue ZIAGEN if hypersensitivity cannot be ruled out, even when other diagnoses are possible. Important information on signs and symptoms of hypersensitivity, as well as clinical management, is presented below. Signs and Symptoms of Hypersensitivity: Hypersensitivity to abacavir is a multi-organ clinical syndrome usually characterized by a sign or symptom in 2 or more of the following groups.
Group 1: Fever Group 2: Rash Group 3: Gastrointestinal (including nausea, vomiting, diarrhea, or abdominal pain) Group 4: Constitutional (including generalized malaise, fatigue, or achiness) Group 5: Respiratory (including dyspnea, cough, or pharyngitis).
Hypersensitivity to abacavir following the presentation of a single sign or symptom has been reported infrequently.
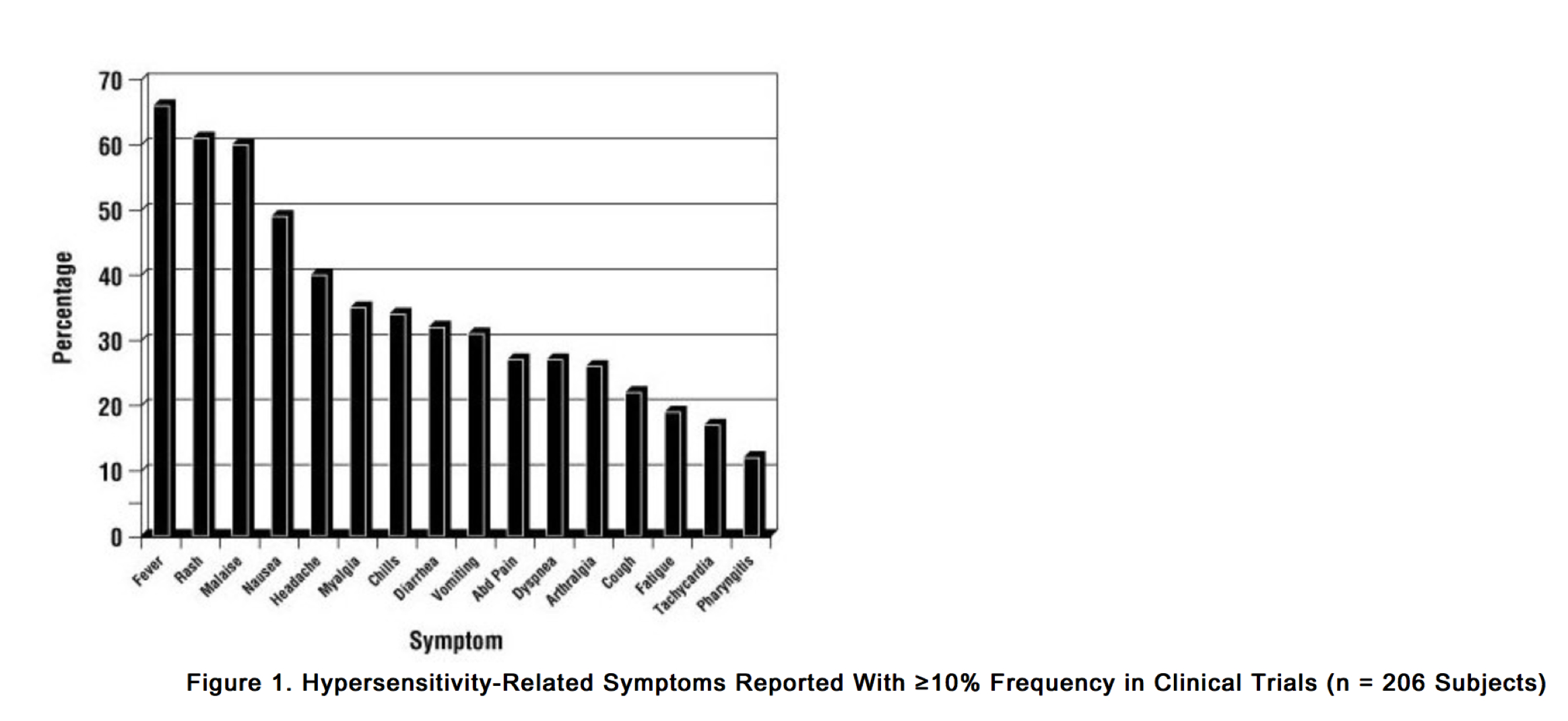
Hypersensitivity to abacavir was reported in approximately 8% of 2,670 subjects (n = 206) in 9 clinical trials (range: 2% to 9%) with enrollment from November 1999 to February 2002. Data on time to onset and symptoms of suspected hypersensitivity were collected on a detailed data collection module. The frequencies of symptoms are shown in Figure 1. Symptoms usually appeared within the first 6 weeks of treatment with abacavir, although the reaction may occur at any time during therapy. Median time to onset was 9 days; 89% appeared within the first 6 weeks; 95% of subjects reported symptoms from 2 or more of the 5 groups listed above.
Other less common signs and symptoms of hypersensitivity include lethargy, myolysis, edema, abnormal chest x-ray findings (predominantly infiltrates, which can be localized), and paresthesia. Anaphylaxis, liver failure, renal failure, hypotension, adult respiratory distress syndrome, respiratory failure, and death have occurred in association with hypersensitivity reactions. In one trial, 4 subjects (11%) receiving ZIAGEN 600 mg once daily experienced hypotension with a hypersensitivity reaction compared with 0 subjects receiving ZIAGEN 300 mg twice daily. Physical findings associated with hypersensitivity to abacavir in some patients include lymphadenopathy, mucous membrane lesions (conjunctivitis and mouth ulcerations), and rash. The rash usually appears maculopapular or urticarial, but may be variable in appearance. There have been reports of erythema multiforme. Hypersensitivity reactions have occurred without rash. Laboratory abnormalities associated with hypersensitivity to abacavir in some patients include elevated liver function tests, elevated creatine phosphokinase, elevated creatinine, and lymphopenia. Clinical Management of Hypersensitivity: Discontinue ZIAGEN as soon as a hypersensitivity reaction is suspected. To minimize the risk of a life-threatening hypersensitivity reaction, permanently discontinue ZIAGEN if hypersensitivity cannot be ruled out, even when other diagnoses are possible (e.g., acute onset respiratory diseases such as pneumonia, bronchitis, pharyngitis, or influenza; gastroenteritis; or reactions to other medications). Following a hypersensitivity reaction to abacavir, NEVER restart ZIAGEN or any other abacavir-containing product because more severe symptoms can occur within hours and may include life-threatening hypotension and death. When therapy with ZIAGEN has been discontinued for reasons other than symptoms of a hypersensitivity reaction, and if reinitiation of ZIAGEN or any other abacavir-containing product is under consideration, carefully evaluate the reason for discontinuation of ZIAGEN to ensure that the patient did not have symptoms of a hypersensitivity reaction. If the patient is of unknown HLA-B*5701 status, screening for the allele is recommended prior to reinitiation of ZIAGEN. If hypersensitivity cannot be ruled out, DO NOT reintroduce ZIAGEN or any other abacavir-containing product. Even in the absence of the HLA-B*5701 allele, it is important to permanently discontinue abacavir and not rechallenge with abacavir if a hypersensitivity reaction cannot be ruled out on clinical grounds, due to the potential for a severe or even fatal reaction. If symptoms consistent with hypersensitivity are not identified, reintroduction can be undertaken with continued monitoring for symptoms of a hypersensitivity reaction. Make patients aware that a hypersensitivity reaction can occur with reintroduction of ZIAGEN or any other abacavir-containing product and that reintroduction of ZIAGEN or any other abacavir-containing product needs to be undertaken only if medical care can be readily accessed by the patient or others. Risk Factor: HLA-B*5701 Allele: Trials have shown that carriage of the HLA-B*5701 allele is associated with a significantly increased risk of a hypersensitivity reaction to abacavir. CNA106030 (PREDICT-1), a randomized, double-blind trial, evaluated the clinical utility of prospective HLA-B*5701 screening on the incidence of abacavir hypersensitivity reaction in abacavir-naive HIV-1-infected adults (n = 1,650). In this trial, use of pre-therapy screening for the HLA-B*5701 allele and exclusion of subjects with this allele reduced the incidence of clinically suspected abacavir hypersensitivity reactions from 7.8% (66/847) to 3.4% (27/803). Based on this trial, it is estimated that 61% of patients with the HLA-B*5701 allele will develop a clinically suspected hypersensitivity reaction during the course of abacavir treatment compared with 4% of patients who do not have the HLA-B*5701 allele. Screening for carriage of the HLA -B*5701 allele is recommended prior to initiating treatment with abacavir. Screening is also recommended prior to reinitiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir. For HLA-B*5701-positive patients, initiating or reinitiating treatment with an abacavir-containing regimen is not recommended and should be considered only with close medical supervision and under exceptional circumstances where potential benefit outweighs the risk. Skin patch testing is used as a research tool and should not be used to aid in the clinical diagnosis of abacavir hypersensitivity. In any patient treated with abacavir, the clinical diagnosis of hypersensitivity reaction must remain the basis of clinical decision-making. Even in the absence of the HLA-B*5701 allele, it is important to permanently discontinue abacavir and not rechallenge with abacavir if a hypersensitivity reaction cannot be ruled out on clinical grounds, due to the potential for a severe or even fatal reaction.
Lactic Acidosis/Severe Hepatomegaly With Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including abacavir and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering ZIAGEN to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with ZIAGEN should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including ZIAGEN. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment. Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Myocardial Infarction
In a published prospective, observational, epidemiological trial designed to investigate the rate of myocardial infarction in patients on combination antiretroviral therapy, the use of abacavir within the previous 6 months was correlated with an increased risk of myocardial infarction (MI).1 In a sponsor-conducted pooled analysis of clinical trials, no excess risk of myocardial infarction was observed in abacavir-treated subjects as compared with control subjects. In totality, the available data from the observational cohort and from clinical trials are inconclusive. As a precaution, the underlying risk of coronary heart disease should be considered when prescribing antiretroviral therapies, including abacavir, and action taken to minimize all modifiable risk factors (e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking).
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
In human liver microsomes, abacavir did not inhibit cytochrome P450 isoforms (2C9, 2D6, 3A4). Based on these data, it is unlikely that clinically significant drug interactions will occur between abacavir and drugs metabolized through these pathways.
Lamivudine and/or Zidovudine
Due to the common metabolic pathways of abacavir and zidovudine via glucuronyl transferase, 15 HIV-1-infected subjects were enrolled in a crossover trial evaluating single doses of abacavir (600 mg), lamivudine (150 mg), and zidovudine (300 mg) alone or in combination. Analysis showed no clinically relevant changes in the pharmacokinetics of abacavir with the addition of lamivudine or zidovudine or the combination of lamivudine and zidovudine. Lamivudine exposure (AUC decreased 15%) and zidovudine exposure (AUC increased 10%) did not show clinically relevant changes with concurrent abacavir.
Ethanol
Due to the common metabolic pathways of abacavir and ethanol via alcohol dehydrogenase, the pharmacokinetic interaction between abacavir and ethanol was studied in 24 HIV-1-infected male subjects. Each subject received the following treatments on separate occasions: a single 600-mg dose of abacavir, 0.7 g/kg ethanol (equivalent to 5 alcoholic drinks), and abacavir 600 mg plus 0.7 g/kg ethanol. Coadministration of ethanol and abacavir resulted in a 41% increase in abacavir AUC∞ and a 26% increase in abacavir t1/2. In males, abacavir had no effect on the pharmacokinetic properties of ethanol, so no clinically significant interaction is expected in men. This interaction has not been studied in females.
Methadone
In a trial of 11 HIV-1-infected subjects receiving methadone-maintenance therapy (40 mg and 90 mg daily), with 600 mg of ZIAGEN twice daily (twice the currently recommended dose), oral methadone clearance increased 22% (90% CI: 6% to 42%). This alteration will not result in a methadone dose modification in the majority of patients; however, an increased methadone dose may be required in a small number of patients. The addition of methadone had no clinically significant effect on the pharmacokinetic properties of abacavir.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Abacavir
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Abacavir is an antiviral agent
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
Pharmacokinetics in Adults
The pharmacokinetic properties of abacavir have been studied in asymptomatic, HIV-1-infected adult subjects after administration of a single intravenous (IV) dose of 150 mg and after single and multiple oral doses. The pharmacokinetic properties of abacavir were independent of dose over the range of 300 to 1,200 mg/day.
Absorption and Bioavailability
Abacavir was rapidly and extensively absorbed after oral administration. The geometric mean absolute bioavailability of the tablet was 83%. After oral administration of 300 mg twice daily in 20 subjects, the steady-state peak serum abacavir concentration (Cmax) was 3.0 ± 0.89 mcg/mL (mean ± SD) and AUC(0-12 hr) was 6.02 ± 1.73 mcg•hr/mL. After oral administration of a single dose of 600 mg of abacavir in 20 subjects, Cmax was 4.26 ± 1.19 mcg/mL (mean ± SD) and AUC∞ was 11.95 ± 2.51 mcg•hr/mL.
Distribution
The apparent volume of distribution after IV administration of abacavir was 0.86 ± 0.15 L/kg, suggesting that abacavir distributes into extravascular space. In 3 subjects, the CSF AUC(0-6 hr) to plasma abacavir AUC(0-6 hr) ratio ranged from 27% to 33%. Binding of abacavir to human plasma proteins is approximately 50%. Binding of abacavir to plasma proteins was independent of concentration. Total blood and plasma drug-related radioactivity concentrations are identical, demonstrating that abacavir readily distributes into erythrocytes.
Metabolism
In humans, abacavir is not significantly metabolized by cytochrome P450 enzymes. The primary routes of elimination of abacavir are metabolism by alcohol dehydrogenase (to form the 5′-carboxylic acid) and glucuronyl transferase (to form the 5′-glucuronide). The metabolites do not have antiviral activity. In vitro experiments reveal that abacavir does not inhibit human CYP3A4, CYP2D6, or CYP2C9 activity at clinically relevant concentrations.
Elimination
Elimination of abacavir was quantified in a mass balance trial following administration of a 600-mg dose of 14C-abacavir: 99% of the radioactivity was recovered, 1.2% was excreted in the urine as abacavir, 30% as the 5′-carboxylic acid metabolite, 36% as the 5′-glucuronide metabolite, and 15% as unidentified minor metabolites in the urine. Fecal elimination accounted for 16% of the dose. In single-dose trials, the observed elimination half-life (t1/2) was 1.54 ± 0.63 hours. After intravenous administration, total clearance was 0.80 ± 0.24 L/hr/kg (mean ± SD). Effects of Food on Oral Absorption: Bioavailability of abacavir tablets was assessed in the fasting and fed states. There was no significant difference in systemic exposure (AUC∞) in the fed and fasting states; therefore, ZIAGEN Tablets may be administered with or without food. Systemic exposure to abacavir was comparable after administration of ZIAGEN Oral Solution and ZIAGEN Tablets. Therefore, these products may be used interchangeably.
Special Populations
Renal Impairment: The pharmacokinetic properties of ZIAGEN have not been determined in patients with impaired renal function. Renal excretion of unchanged abacavir is a minor route of elimination in humans.
Hepatic Impairment
The pharmacokinetics of abacavir have been studied in subjects with mild hepatic impairment (Child-Pugh score 5 to 6). Results showed that there was a mean increase of 89% in the abacavir AUC and an increase of 58% in the half-life of abacavir after a single dose of 600 mg of abacavir. The AUCs of the metabolites were not modified by mild liver disease; however, the rates of formation and elimination of the metabolites were decreased. A dose of 200 mg (provided by 10 mL of ZIAGEN Oral Solution) administered twice daily is recommended for patients with mild liver disease. The safety, efficacy, and pharmacokinetics of abacavir have not been studied in patients with moderate or severe hepatic impairment; therefore, ZIAGEN is contraindicated in these patients.
Pediatric Patients
The pharmacokinetics of abacavir have been studied after either single or repeat doses of ZIAGEN in 68 pediatric subjects. Following multiple-dose administration of ZIAGEN 8 mg/kg twice daily, steady-state AUC(0-12 hr) and Cmax were 9.8 ± 4.56 mcg•hr/mL and 3.71 ± 1.36 mcg/mL (mean ± SD), respectively [see Use in Specific Populations (8.4)]. In addition, to support dosing of ZIAGEN scored tablet (300 mg) for pediatric patients 14 kg to greater than 30 kg, analysis of actual and simulated pharmacokinetic data indicated comparable exposures are expected following administration of 300 mg scored tablet and the 8 mg/kg dosing regimen using oral solution. Geriatric Patients: The pharmacokinetics of ZIAGEN have not been studied in patients over 65 years of age. Gender: A population pharmacokinetic analysis in HIV-1-infected male (n = 304) and female (n = 67) subjects showed no gender differences in abacavir AUC normalized for lean body weight.
Race
There are no significant differences between blacks and Caucasians in abacavir pharmacokinetics.
Nonclinical Toxicology
Microbiology
Abacavir is a carbocyclic synthetic nucleoside analogue. Abacavir is converted by cellular enzymes to the active metabolite, carbovir triphosphate (CBV-TP), an analogue of deoxyguanosine-5′-triphosphate (dGTP). CBV-TP inhibits the activity of HIV-1 reverse transcriptase (RT) both by competing with the natural substrate dGTP and by its incorporation into viral DNA. The lack of a 3′-OH group in the incorporated nucleotide analogue prevents the formation of the 5′ to 3′ phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated. CBV-TP is a weak inhibitor of cellular DNA polymerases α, β, and γ.
Antiviral Activity
The antiviral activity of abacavir against HIV-1 was evaluated against a T-cell tropic laboratory strain HIV-1IIIB in lymphoblastic cell lines, a monocyte/macrophage tropic laboratory strain HIV-1BaL in primary monocytes/macrophages, and clinical isolates in peripheral blood mononuclear cells. The concentration of drug necessary to effect viral replication by 50 percent (EC50) ranged from 3.7 to 5.8 μM (1 μM = 0.28 mcg/mL) and 0.07 to 1.0 μM against HIV-1IIIB and HIV-1BaL, respectively, and was 0.26 ± 0.18 μM against 8 clinical isolates. The EC50values of abacavir against different HIV-1 clades (A-G) ranged from 0.0015 to 1.05 μM, and against HIV-2 isolates, from 0.024 to 0.49 μM. The antiviral activity of abacavir in cell culture was not antagonized when combined with the nucleoside reverse transcriptase inhibitors (NRTIs) didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine or zidovudine, the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine, or the protease inhibitor (PI) amprenavir. Ribavirin (50 μM) had no effect on the anti–HIV-1 activity of abacavir in cell culture.
Resistance
HIV-1 isolates with reduced susceptibility to abacavir have been selected in cell culture and were also obtained from subjects treated with abacavir. Genotypic analysis of isolates selected in cell culture and recovered from abacavir-treated subjects demonstrated that amino acid substitutions K65R, L74V, Y115F, and M184V/I in RT contributed to abacavir resistance. In a trial of therapy-naive adults receiving ZIAGEN 600 mg once daily (n = 384) or 300 mg twice daily (n = 386), in a background regimen of lamivudine 300 mg once daily and efavirenz 600 mg once daily (CNA30021), the incidence of virologic failure at 48 weeks was similar between the 2 groups (11% in both arms). Genotypic (n = 38) and phenotypic analyses (n = 35) of virologic failure isolates from this trial showed that the RT substitutions that emerged during abacavir once-daily and twice-daily therapy were K65R, L74V, Y115F, and M184V/I. The substitution M184V/I was the most commonly observed substitution in virologic failure isolates from subjects receiving abacavir once daily (56%, 10/18) and twice daily (40%, 8/20).
Thirty-nine percent (7/18) of the isolates from subjects who experienced virologic failure in the abacavir once-daily arm had a greater than 2.5-fold decrease in abacavir susceptibility with a median-fold decrease of 1.3 (range: 0.5 to 11) compared with 29% (5/17) of the failure isolates in the twice-daily arm with a median-fold decrease of 0.92 (range: 0.7 to 13).
Cross-Resistance
Cross-resistance has been observed among NRTIs. Isolates containing abacavir resistance-associated substitutions, namely, K65R, L74V, Y115F, and M184V, exhibited cross-resistance to didanosine, emtricitabine, lamivudine, tenofovir, and zalcitabine in cell culture and in subjects. The K65R substitution can confer resistance to abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zalcitabine; the L74V substitution can confer resistance to abacavir, didanosine, and zalcitabine; and the M184V substitution can confer resistance to abacavir, didanosine, emtricitabine, lamivudine, and zalcitabine. An increasing number of thymidine analogue mutations (TAMs: M41L, D67N, K70R, L210W, T215Y/F, K219E/R/H/Q/N) is associated with a progressive reduction in abacavir susceptibility.[1]
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Abacavir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Abacavir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Abacavir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Abacavir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Abacavir Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "ZIAGEN (ABACAVIR SULFATE) TABLET, FILM COATED ZIAGEN (ABACAVIR SULFATE) SOLUTION [VIIV HEALTHCARE COMPANY]". Retrieved 30 December 2013.