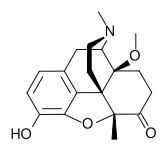
14-Methoxymetopon
 | |
| Clinical data | |
|---|---|
| Synonyms | 14-Methoxymetopon |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H23NO4 |
| Molar mass | 329.39 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
14-Methoxymetopon is an opiate analogue that is an derivative of metopon which has been substituted with a methoxy group at the 14-position. It is a highly potent analgesic drug that is around 500x stronger than morphine when administered systemically; however, when given spinally, it is up to 1 million times the potency of morphine.[1] It binds strongly to the μ-opioid receptor and activates it to a greater extent than most similar opioid drugs.[2] This produces an unusual pharmacological profile, and although 14-methoxymetopon acts as a potent μ-opioid full agonist in regards to some effects such as analgesia, a ceiling effect is seen on other effects such as constipation and respiratory depression.[3]
References
- ↑ King MA, Su W, Nielan CL, Chang AH, Schütz J, Schmidhammer H, Pasternak GW. 14-Methoxymetopon, a very potent mu-opioid receptor-selective analgesic with an unusual pharmacological profile. European Journal of Pharmacology. 2003 Jan 17;459(2-3):203-9. PMID 12524147
- ↑ Mahurter L, Garceau C, Marino J, Schmidhammer H, Tóth G, Pasternak GW. Separation of binding affinity and intrinsic activity of the potent mu-opioid 14-methoxymetopon. Journal of Pharmacology and Experimental Therapeutics. 2006 Oct;319(1):247-53. PMID 16801454
- ↑ Freye E, Schmidhammer H, Latasch L. 14-methoxymetopon, a potent opioid, induces no respiratory depression, less sedation, and less bradycardia than sufentanil in the dog. Anesthesia and Analgesia. 2000 Jun;90(6):1359-64. PMID 10825321
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Opioids