Tirofiban
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tirofiban is a Platelet Aggregation Inhibitor that is FDA approved for the {{{indicationType}}} of reduce the rate of thrombotic cardiovascular events. Common adverse reactions include Bradyarrhythmia, Bleeding, Pain in pelvis (6% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Reduction the rate of thrombotic cardiovascular events
- Dosing information
- 25 mcg/kg IV over 3 minutes and then 0.15 mcg/kg/min (or 0.075 mcg/kg/min for patients with serum creatinine ≤60 mL/min), for up to 18 hours. This is not the regimen that was used in studies that established effectiveness of AGGRASTAT [see Clinical Studies (14)].
- The instructions by weight and creatinine clearance are tabulated in Table 1.

- Important Administration Instructions
- To open the container, first tear off its foil overpouch. The plastic may be somewhat opaque because of moisture absorption during sterilization; the opacity will diminish gradually. Check for leaks by squeezing the inner bag firmly; if any leaks are found or sterility is suspect then the solution should be discarded. Do not use unless the solution is clear and the seal is intact. Suspend the container from its eyelet support, remove the plastic protector from the outlet port, and attach a conventional administration set.
- You may administer AGGRASTAT in the same intravenous line as atropine sulfate, dobutamine, dopamine, epinephrine hydrochloride (HCl), famotidine injection, furosemide, lidocaine, midazolam HCl, morphine sulfate, nitroglycerin, potassium chloride, and propranolol HCl. :* :* Do not administer AGGRASTAT through the same IV line as diazepam.
- Do not add other drugs or remove solution directly from the bag with a syringe.
- Do not use plastic containers in series connections; such use can result in air embolism by drawing air from the first container if it is empty of solution.
- Discard any unused portion left in the bag.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tirofiban sandbox in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tirofiban sandbox in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tirofiban sandbox in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tirofiban sandbox in pediatric patients.
Contraindications
- Severe hypersensitivity reaction to AGGRASTAT (i.e., anaphylactic reactions) [see Adverse Reactions (6.2)].
- A history of thrombocytopenia following prior exposure to AGGRASTAT [see Adverse Reactions (6.1)].
- Active internal bleeding or a history of bleeding diathesis, major surgical procedure or severe physical trauma within the previous month [see Adverse Reactions (6.1)]
Warnings
General Risk of Bleeding
Bleeding is the most common complication encountered during therapy with AGGRASTAT. Most bleeding associated with AGGRASTAT occurs at the arterial access site for cardiac catheterization. Minimize the use of traumatic or potentially traumatic procedures such arterial and venous punctures, intramuscular injections, nasotracheal intubation, etc. Fatal bleeding events have been reported [see Adverse Reactions (6.2)].
Concomitant use offibrinolytics, oral anticoagulants and antiplatelet drugs increases the risk of bleeding.
Thrombocytopenia
Profound thrombocytopenia has been reported with AGGRASTAT. Monitor platelet counts beginning about 6 hours after treatment initiation and daily thereafter. If the platelet count decreases to <90,000/mm3, monitor platelet counts to exclude pseudothrombocytopenia. If thrombocytopenia is confirmed, discontinue AGGRASTAT and heparin. Previous exposure to a glycoprotein (GP) IIb/IIIa receptor antagonist may increase the risk of developing thrombocytopenia [see Adverse Reactions (6.1)].
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In the PRISM (Platelet Receptor Inhibition for Ischemic Syndrome Management), PRISM-PLUS (Platelet Receptor Inhibition for Ischemic Syndrome Management — Patients Limited by Unstable Signs and Symptoms) and RESTORE (Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis) trials, 1946 patients received AGGRASTAT in combination with heparin and 2002 patients received AGGRASTAT alone for about 3 days. Forty-three percent of the population was >65 years of age and approximately 30% of patients were female. In clinical studies with the recommended regimen (25 mcg/kg bolus followed by a 0.15 mcg/kg/min maintenance infusion), AGGRASTAT was administered in combination with aspirin, clopidogrel and heparin or bivalirudin to over 8000 patients for typically ≤24 hours. Approximately 30% of the population was >65 years of age and approximately 25% were female.
Bleeding
PRISM-PLUS Regimen
The incidences of major and minor bleeding using the TIMI criteria in the PRISM-PLUS study are shown below.
{
Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of AGGRASTAT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
Hypersensitivity: Severe allergic reactions including anaphylactic reactions have occurred during the first day of AGGRASTAT infusion, during initial treatment, and during readministration of AGGRASTAT. Some cases have been associated with severe thrombocytopenia (platelet counts <10,000/mm3). No information is available on the formation of antibodies to tirofiban.
Drug Interactions
Use ofthrombolytics, anticoagulants, and other antiplatelet agents.
Coadministration of antiplatelet agents, thrombolytics, heparin, and aspirin increases the risk of bleeding.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
There are no adequate and well-controlled studies in pregnant women. Tirofiban has been shown to cross the placenta in pregnant rats and rabbits. Studies with tirofiban HCl at intravenous doses up to 5 mg/kg/day (about 5 and 13 times the maximum recommended daily human dose for rat and rabbit, respectively, when compared on a body surface area basis) have revealed no harm to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tirofiban in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tirofiban during labor and delivery.
Nursing Mothers
It is not known whether tirofiban is excreted in human milk. However, significant levels of tirofiban were shown to be present in rat milk. Because many drugs are excreted in human milk, and because of the potential for adverse effects on the nursing infant, discontinue nursing or discontinue AGGRASTAT.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Of the total number of patients in controlled clinical studies of AGGRASTAT, 43% were 65 years and over, while 12% were 75 and over. With respect to efficacy, the effect of AGGRASTAT in the elderly (≥65 years) appeared similar to that seen in younger patients (<65 years). Elderly patients receiving AGGRASTAT with heparin or heparin alone had a higher incidence of bleeding complications than did younger patients, but the incremental risk of bleeding in patients treated with AGGRASTAT in combination with heparin compared to the risk in patients treated withheparin alone was similar regardless of age. No dose adjustment is recommended for the elderly population [see Dosage and Administration (2)].
Gender
There is no FDA guidance on the use of Tirofiban with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tirofiban with respect to specific racial populations.
Renal Impairment
Patients with moderate to severerenal insufficiency have decreased plasma clearance of AGGRASTAT. Reduce the dosage of AGGRASTAT in patients with severe renal insufficiency [see Dosage and Administration (2) and Clinical Pharmacology (12.3)].
Safety and efficacy of AGGRASTAT has not been established in patients on hemodialysis.
Hepatic Impairment
There is no FDA guidance on the use of Tirofiban in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tirofiban in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tirofiban in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tirofiban Administration in the drug label.
Monitoring
There is limited information regarding Tirofiban Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tirofiban and IV administrations.
Overdosage
In clinical trials, inadvertent overdosage with AGGRASTAT occurred in doses up to 2 times the recommended dose for initial infusion doses. Inadvertent overdosage occurred in doses up to 9.8 times the 0.15 mcg/kg/min maintenance infusion rate.
The most frequently reported manifestation of overdosage was bleeding, primarily minor mucocutaneous bleeding events and minor bleeding at the sites of cardiac catheterization [see WARNINGS AND PRECAUTIONS (5.1)].
Overdosage of AGGRASTAT should be treated by assessment of the patient’s clinical condition and cessation or adjustment of the drug infusion as appropriate.
AGGRASTAT can be removed by hemodialysis.
Pharmacology
| |
| |
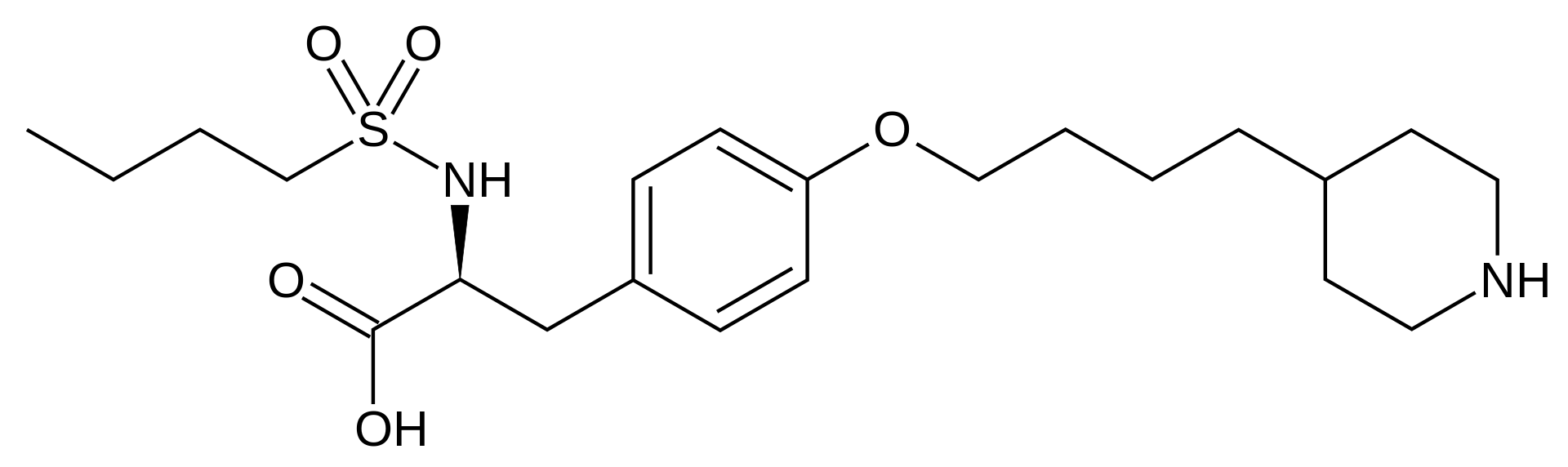
Tirofiban
| |
| Systematic (IUPAC) name | |
| (S)-2-(butylsulfonamino)-3-(4-[4-(piperidin-4-yl)butoxy]phenyl)propanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 440.598 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | n/a (IV only) |
| Protein binding | 65% |
| Metabolism | ? |
| Half life | 2 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | Exclusively intravenous |
Mechanism of Action
AGGRASTAT is a reversible antagonist of fibrinogen binding to the GP IIb/IIIa receptor, the major platelet surface receptor involved in platelet aggregation. When administered intravenously, AGGRASTAT inhibits ex vivo platelet aggregation in a dose- and concentration-dependent manner.
When given according to the PRISM-PLUS regimen of 0.4 mcg/kg/min for 30 minutes followed by a 0.1 mcg/kg/min maintenance infusion, >90% inhibition of platelet aggregation is attained by the end of the 30-minute infusion. When given according to the recommended regimen of 25 mcg/kg over 3 min followed by a 0.15 mcg/kg/min maintenance infusion, >90% inhibition of platelet aggregation is attained within 10 minutes. Platelet aggregation inhibition is reversible following cessation of the infusion of AGGRASTAT.
Structure
AGGRASTAT contains tirofiban hydrochloride, a non-peptide antagonist of the platelet GP IIb/IIIa receptor, inhibits platelet aggregation.
Tirofiban hydrochloride monohydrate is chemically described as N(butylsulfonyl)-O-[4-(4-piperidinyl)butyl]-L-tyrosine monohydrochloride monohydrate.
Its molecular formula is C22H36N2O5S•HCl•H2O and its structural formula is:


Tirofiban hydrochloride monohydrate is a white to off-white, non-hygroscopic, free-flowing powder, with a molecular weight of 495.08. It is very slightly soluble in water.
AGGRASTAT Injection Premixed is supplied as a sterile solution in water for injection, for intravenous use only, in plastic containers of 100 mL or 250 mL. Each 100 mL of the premixed, iso-osmotic intravenous injection contains 5.618 mg tirofiban hydrochloride monohydrate equivalent to 5 mg tirofiban (50 mcg/mL) and the following inactive ingredients: 0.9 g sodium chloride, 54 mg sodium citrate dihydrate, and 3.2 mg citric acid anhydrous. Each 250 mL of the premixed, iso-osmotic intravenous injection contains 14.045 mg tirofiban hydrochloride monohydrate equivalent to 12.5 mg tirofiban (50 mcg/mL) and the following inactive ingredients: 2.25 g sodium chloride, 135 mg sodium citrate dihydrate, and 8 mg citric acid anhydrous.
The pH of the solution ranges from 5.5 to 6.5 and may have been adjusted with hydrochloric acid and/or sodium hydroxide. The flexible container is manufactured from a specially designed multilayer plastic (PL 2408). Solutions in contact with the plastic container leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
Pharmacodynamics
AGGRASTAT inhibits platelet function, as demonstrated by its ability to inhibit ex vivo adenosine phosphate (ADP)-induced platelet aggregation and prolong bleeding time in healthy subjects and patients with coronary artery disease. The time course of inhibition parallels the plasma concentration profile of the drug.
Following discontinuation of an infusion of AGGRASTAT 0.10 mcg/kg/min, ex vivo platelet aggregation returns to near baseline in 4 to 8 hours in approximately 90% of patients with coronary artery disease. The addition of heparin to this regimen does not significantly alter the percentage of subjects with >70% inhibition of platelet aggregation (IPA), but does increase the average bleeding time, as well as the number of patients with bleeding times prolonged to >30 minutes. Similar platelet aggregation recovery rates are observed following discontinuation of a 0.15 mcg/kg/min infusion.
Pharmacokinetics
Tirofiban has a half-life of approximately 2 hours. It is cleared from the plasma largely by renal excretion, with about 65% of an administered dose appearing in urine and about 25% in feces, both largely as unchanged tirofiban. Metabolism appears to be limited.
Tirofiban is not highly bound to plasma proteins and protein binding is concentration independent over the range of 0.01 to 25 mcg/mL. The unbound fraction in human plasma is 35%. The steady state volume of distribution of tirofiban ranges from 22 to 42 liters.
In healthy subjects, the plasma clearance of tirofiban ranges from 213 to 314 mL/min. Renal clearance accounts for 39 to 69% of plasma clearance.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of AGGRASTAT has not been evaluated.
Tirofiban HCI was negative in the in vitro microbial mutagenesis and V-79 mammalian cell mutagenesis assays. In addition, there was no evidence of direct genotoxicity in the in vitro alkaline elution and in vitro chromosomal aberration assays. There was no induction of chromosomal aberrations in bone marrow cells of male mice after the administration of intravenous doses up to 5 mg tirofiban/kg (about 3 times the maximum recommended daily human dose when compared on a body surface area basis).
Fertility and reproductive performance were not affected in studies with male and female rats given intravenous doses of tirofiban up to 5 mg/kg/day (about 5 times the maximum recommended daily human dose when compared on a body surface area basis).
Clinical Studies
There is limited information regarding Tirofiban Clinical Studies in the drug label.
How Supplied
There is limited information regarding Tirofiban How Supplied in the drug label.
Storage
There is limited information regarding Tirofiban Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tirofiban |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tirofiban |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tirofiban Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Tirofiban sandbox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tirofiban Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tirofiban Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.