Oesophagostomum natural history, complications and prognosis
|
Oesophagostomum Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Oesophagostomum natural history, complications and prognosis On the Web |
|
American Roentgen Ray Society Images of Oesophagostomum natural history, complications and prognosis |
|
FDA onOesophagostomum natural history, complications and prognosis |
|
CDC on Oesophagostomum natural history, complications and prognosis |
|
on Oesophagostomum natural history, complications and prognosis |
|
Risk calculators and risk factors for Oesophagostomum natural history, complications and prognosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please help WikiDoc by adding more content here. It's easy! Click here to learn about editing.
Natural History
Transmission
Transmission of Oesophagostomum is believed to be oral-fecal for both humans and animals, largely because percutaneous infection with Oesophagostomum has never been reported.[1] It is unclear whether or not parasite transmission is specifically waterborne, foodborne, or both. Regardless, introduction of the stage three infective larvae is necessary for human infection. Much about the biological mechanism of transmission is still unknown, and current knowledge of oral-fecal transmission mechanisms does not explain why Oesophagostomum are mostly localized to Northern Togo and Ghana. It is possible that there are behavioral factors or unique soil conditions that facilitate larval development and are not found outside the current endemic areas.[2] Oesophagostomiasis is generally classified as a zoonotic disease, which is an infectious disease that can be transmitted between animals and humans. This has been called into question recently, as recent research has found that human-to-human transmission is possible.[3]
Reservoir
Oesophagostomum are carried predominantly by non-humans, infecting cattle, sheep, goats, wild pigs, and primates. Humans are largely presumed to be an accidental host, as they are not suitable for completion of the Oesophagostomum development; however, the extreme localization of oesophagostomiasis to northern Togo and Ghana in Africa suggests the possibility that the Oesophagostomum is increasingly exhibiting preference for human hosts.[4]
Until recently it was believed that primates were the main reservoirs of human-infecting Oesophagostomum in northern Togo and Ghana, as these particular species have a considerable concentration in non-human primate reservoirs. A 2005 study done by van Lieshout and de Grujiter found that O. bifurcum in humans from northern Ghana is distinct from the O. bifurcum found in olive baboons and mona monkeys outside the endemic area. They used species-specific PCR and microscopy to establish the identification of two separate species of O. bifurcum. [5] These results are significant, as they necessitate further research to determine the definitive reservoirs of human-infecting O. bifurcum.
Vector
Oesophagostomiasis has no vector.[6]
Incubation period
The life-cycle of Oesophagostomum can usually be completed in less than 60 days.[7] When the eggs are passed into the feces to the outside environment, they hatch into stage one larve. The stage two larve then molt twice, developing into infective stage three larva in 6–7 days. These stage three larvae can survive extended periods of desiccation by shrinking within their sheaths.[8]
Morphology
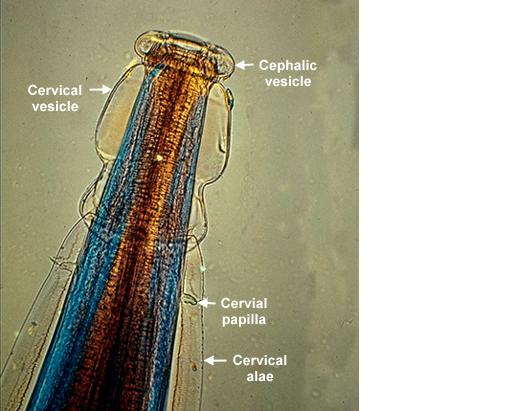
Adult worms of all Oesophagostomum spp. exhibit a cephalic groove by its proximal gut as well as a visible secretory pore, or stomum, at the same level of the oesophagus19. Like other nematodes, Oesophagostomum spp. contain a developed, multi-nucleate digestive tract as well as a reproductive system. Their developed buccal capsule and club-shaped oesophagus are useful for distinguishing Oesophagostomum spp. from hookworms.[9]
Both sexes of adults have a cephalic inflation and an oral opening lined with both internal and external leaf crowns.[10] Female adults, which have a length range of 6.5–24 mm, are generally larger than their male counterparts, with a length range of 6-16.6 mm. Males can be distinguished by their bell-like copulatory bursa, located in the tail, and their paired rodlike spicules.[11]
Eggs are ovular in shape and range from 50 to 100 micrometres in size; they closely resembles those of hookworms, which renders diagnosis via stool analysis useless in areas co-infected with both Oesophagostomum and hookworm.[12]
Life cycle
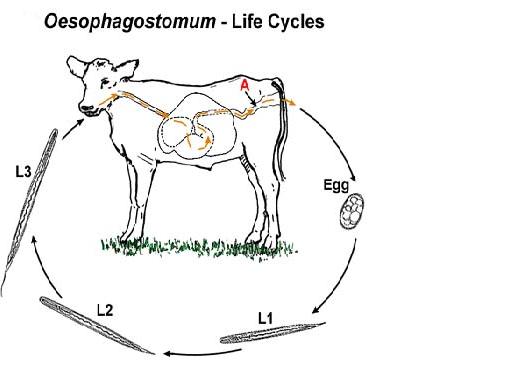
For non-human hosts, the life cycle of Oesophagostomum begins with the passing of eggs in the animal feces. From there the eggs develop into stage one larvae. These larvae then spend 6–7 days in the environment developing into stage two and then infectious stage three larvae.[13] Infection begins with the ingestion of soil contaminated with stage three larvae. After ingestion the larvae end up in the small intestine, unsheathing and penetrating the intestinal wall to form nodules. The resulting adult worms that remain in the intestinal lumen copulate; the eggs from the female are then deposited in the feces. Females usually lay around 5,000 eggs per day, which is on par with reproductive rates of other nematodes within Strongyloidea.[14]
For human hosts, the life cycle is very similar to that of Oesophagostomum in animals. It begins when an animal reservoir defecates into the soil, leaving feces infested with eggs that develop into rhabitiform larvae.[15] These larve then develop into stage two and then infectious stage three larvae in the environment over the course of 6–7 days. Human infection occurs when soil or water containing the third-stage larvae is ingested, presumably via contaminated meat obtained from infected livestock or crops with contaminated soil. Once ingested, the filariform larvae migrate to the submucosa of the small or large intestine, then to the lumen of the colon. The developing worms then penetrate the intestinal tissues, causing nodular lesion formation in the intestines and colon; it is in these nodules that the larvae mature to stage four larvae.[4] These larvae may then emerge from their nodules and migrate back to the intestinal lumen, where they mature into adults. But many larvae often do not complete development and remain in their colon nodules, as humans are generally unsuitable hosts for Oesophagostomum. The instances where Oesophagostomumhave completed development in humans seem to be dependent on certain environmental and host factors that have yet to be identified.[16]
References
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑
- ↑ 4.0 4.1 Gasser, R B, J M de Gruijter, and A M Polderman. “Insights into the epidemiology and genetic make-up of Oesophagostomum bifurcum from human and non-human primates using molecular tools.” Parasitology 132.Pt 4 (2006): 453-60.
- ↑ van Lieshout, Lisette et al. “Oesophagostomum bifurcum in non-human primates is not a potential reservoir for human infection in Ghana.” Tropical Medicine & International Health: TM & IH 10.12 (2005): 1315-20.
- ↑ “GIDEON Infectious Diseases - Diseases.” GIDEON Infectious Disease Database. 5 Feb 2009.<http://web.gideononline.com/web/epidemiology/index.php?gdn_form=ZGlzZWFzZT0xMTY1MA==>.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑
- ↑ Elmes, B et al. (1953). Helminthic abscess, a surgical complication of oesophagostomes and hookworms. Annals of Tropical Medicine and Parasitology. 48: 1-7.
- ↑
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Krepel, H P, and A M Polderman. “Egg production of Oesophagostomum bifurcum, a locally common parasite of humans in Togo.” The American Journal of Tropical Medicine and Hygiene 46.4 (1992): 469-72.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Ziem, J.B. et al. “Impact of repeated mass treatment on human Oesophagostomum and hookworm infections in northern Ghana.” Tropical Medicine & International Health: TM & IH 11.11 (2006): 1764-72.