Brucellosis pathophysiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief:
|
Brucellosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Brucellosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Brucellosis pathophysiology |
|
Risk calculators and risk factors for Brucellosis pathophysiology |
Overview
Brucella is usually transmitted via the digestive route to the human host. Following transmission, white blood cells phagocyte the pathogen and transports it via the hematologic or lymphatic route to different organs, specially to those of the reticuloendothelial system.[1][2]
Pathophysiology
Brucellosis is a zoonotic disease, Humans could be infected by eating undercook meat or raw dairy products, inhalation of the bacteria, and direct contact bacteria with skin wounds or mucous membranes. Following transmission, white blood cells phagocyte the pathogen and transports it via the hematologic or lymphatic route to different organs, specially to those of the reticuloendothelial system. Endotoxic lipopolysaccharide LPS, plays an important role in survival of bacteria inside monocytic cell, suprresing-lysosome fusion, and Internalizing bacteria into endoplasmic reticulum. The pathophysiology of brucellosis may be described in the following steps:[1][2][3][4][5][6][7][8][9][10][11][12][13]
Transmissio
| Humans are generally infected with brucellosis in one of three ways | |
|---|---|
Eating undercooked meat or consuming unpasteurized/raw dairy products |
The most common way to be infected is by eating or drinking unpasteurized/raw dairy products. When sheep, goats, cows, or camels are infected, their milk becomes contaminated with the bacteria.
If the milk from infected animals is not pasteurized, the infection will be transmitted to people who consume the milk and/or cheese products. |
Breathing in the bacteria that cause brucellosis (inhalation) |
Breathing in the bacteria that causes brucellosis may also lead to infection. This risk is generally greater for people in laboratories that work with the bacteria. In addition, slaughterhouse and meat-packing employees have also been known to be exposed to the bacteria and ultimately become infected. |
Bacteria entering the body through skin wounds or mucous membranes |
Bacteria can also enter wounds in the skin/mucous membranes through contact with infected animals.
This poses a problem for workers who have close contact with animals or animal excretions (newborn animals, fetuses, and excretions that may result from birth). Such workers may include:
|
| |
Incubation
Incubation period of brucellosis varies from one to four weeks. But occasionally, it may be as long as several months. [3][4]
Dissemination
Following transmission, brucellae is ingested by macrophages and polymorphonuclear cells. On ingestion, they replicate intracellularly inside the lysed cells, infecting the other cells or disseminating systemically. [5]
Seeding
- On transmission, Neutrophilic granulocytes and monocytes actively phagocytosed the bacteria
- On entry into the body, brucellae multiply in the neutrophilic granulocytes ad monocytes, initially in lymph nodes, which is followed by systemic hematogenous spread resulting in multiple localizing infection
Immune response
Brucellosis elicits both humoral and cell-mediated immune responses:[14]
Humoral immune response
Humoral response has a limited role in protecting host from Brucellae. On activation, B-cell produce IgM class antibody, which is followed by IgG antibodies . Antibodies promote clearance of extracellular and facitlitate phagocytosis of the Brucellae.
Cell mediates immune response
- Tumor necrosis factor α (TNF-α) produce on activation of cell mediated immunity, stimulates T lymphocytes and macrophages, which help in eliminating intracellular brucellae. Virulent brucellar tend to supress the activity of Tumor necrosis factor α (TNF-α) and IFN-gamma
- Cytokines such as interleukin (IL) 12 promote production of Interferon γ (IFN-γ) responses. IFN-γ, which drives TH1-type responses and stimulates macrophage activation. Cytokines, which include IL-4, IL-6, and IL-10, downregulate the protective response.
Pathogenesis
The pathogenesis of brucellosis involve the following:[15][16]
By avoiding innate immunity, brucella survive with in monocytic cells.
- Endotoxic lipopolysaccharide LPS, plays a key role in survival of bacteria inside monocytic cell.
- LPS helps in survival of the bacteria inside the monocytic cell, by suppressing phagosome–lysosome fusion, internalizing bacteria into endoplasmic reticulum and inhibiting apoptosis of infected cell.
- Type IV secretion system (VirB) and type III secretion system, that regulates intracellular survival and trafficking has been identified, although type 3 not yet confirmed.
- Acid-stable proteins produced by brucella, facilitates the survival in phogosomes
- Cu-Zn superoxide dismutase, produced by brucellae, gives them resistance from reactive oxygen intermediates.
Genetics
There is no known genetic association to brucellosis.
Microscopic Pathology
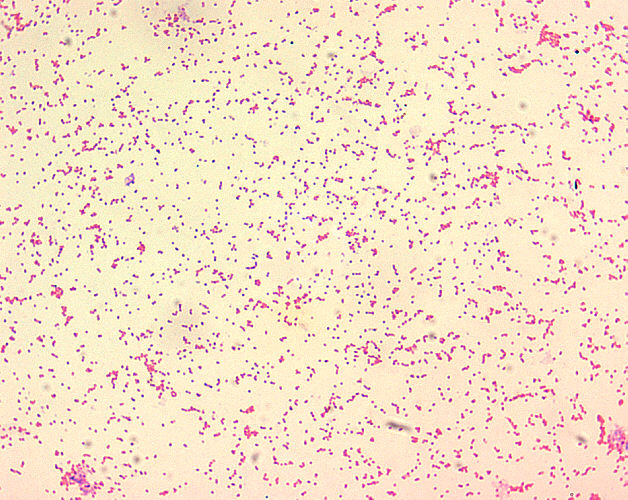
- Brucella spp. are poorly staining, small gram-negative coccobacilli (0.5-0.7 x 0.6-1.5 µm).
- Brucella spp. are seen mostly as single cells and appearing like “fine sand”.[17]
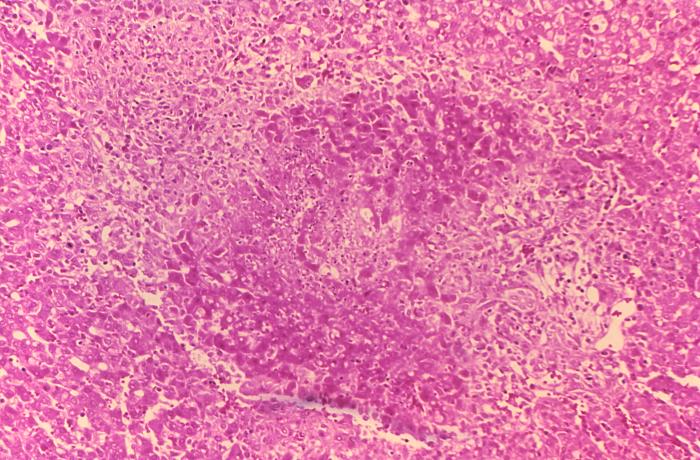
- On microscopic histopathological analysis of the liver, common findings are:
- Granulomas with centrilobular necrosis or focal necrosis and parenchyma destruction.[18]
- **
-
Brucella spp. are poorly staining, small gram-negative coccobacilli (0.5-0.7 x 0.6-1.5 µm), and are seen mostly as single cells and appearing like “fine sand”.
-
Histopathology of guinea pig liver in experimental Brucella suis infection. Granuloma with necrosis.
Reference
- ↑ Pappas G, Akritidis N, Bosilkovski M, Tsianos E (2005). "Brucellosis". N Engl J Med. 352 (22): 2325–36. doi:10.1056/NEJMra050570. PMID 15930423.
- ↑ "CDC".
- ↑ Zhan Y, Liu Z, Cheers C (1996). "Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms". Infect Immun. 64 (7): 2782–6. PMC 174139. PMID 8698508.
- ↑ Larralde de Luna M, Raspa ML, Ibargoyen J (1992). "Oral-facial-digital type 1 syndrome of Papillon-Léage and Psaume". Pediatr Dermatol. 9 (1): 52–6. PMID 1574477.
- ↑ Gazapo E, Gonzalez Lahoz J, Subiza JL, Baquero M, Gil J, de la Concha EG (1989). "Changes in IgM and IgG antibody concentrations in brucellosis over time: importance for diagnosis and follow-up". J Infect Dis. 159 (2): 219–25. PMID 2915152.
- ↑ Arenas GN, Staskevich AS, Aballay A, Mayorga LS (2000). "Intracellular trafficking of Brucella abortus in J774 macrophages". Infect Immun. 68 (7): 4255–63. PMC 101738. PMID 10858243.
- ↑ Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A; et al. (2002). "Type IV secretion and Brucella virulence". Vet Microbiol. 90 (1–4): 341–8. PMID 12414154.
- ↑ Lapaque N, Moriyon I, Moreno E, Gorvel JP (2005). "Brucella lipopolysaccharide acts as a virulence factor". Curr Opin Microbiol. 8 (1): 60–6. doi:10.1016/j.mib.2004.12.003. PMID 15694858.
- ↑ DelVecchio VG, Kapatral V, Elzer P, Patra G, Mujer CV (2002). "The genome of Brucella melitensis". Vet Microbiol. 90 (1–4): 587–92. PMID 12414174.
- ↑ Moreno E, Moriyon I (2002). "Brucella melitensis: a nasty bug with hidden credentials for virulence". Proc Natl Acad Sci U S A. 99 (1): 1–3. doi:10.1073/pnas.022622699. PMC 117501. PMID 11782541.
- ↑ Gorvel JP, Moreno E (2002). "Brucella intracellular life: from invasion to intracellular replication". Vet Microbiol. 90 (1–4): 281–97. PMID 12414149.
- ↑ Ko J, Splitter GA (2003). "Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans". Clin Microbiol Rev. 16 (1): 65–78. PMC 145300. PMID 12525425.
- ↑ Dornand J, Gross A, Lafont V, Liautard J, Oliaro J, Liautard JP (2002). "The innate immune response against Brucella in humans". Vet Microbiol. 90 (1–4): 383–94. PMID 12414158.
- ↑ Khan M, Harms JS, Marim FM, Armon L, Hall CL, Liu YP; et al. (2016). "The Bacterial Second Messenger Cyclic di-GMP Regulates Brucella Pathogenesis and Leads to Altered Host Immune Response" Check
|url=value (help). Infect Immun. 84 (12): 3458–3470. doi:10.1128/IAI.00531-16. PMC 5116723. PMID 27672085. - ↑ Barquero-Calvo E, Chaves-Olarte E, Weiss DS, et al. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS One 2007; 2:e631.
- ↑ Gorvel JP, Moreno E. Brucella intracellular life: from invasion to intracellular replication. Vet Microbiol 2002; 90:281.
- ↑ Brucellosis. Wikipedia. https://en.wikipedia.org/wiki/Brucellosis. Accessed on January 29, 2016
- ↑ Hunt A, Bothwell P. Histological findings in human brucellosis. J Clin Pathol. 1967; 20: 267-272