Cefprozil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cefprozil is a 2nd generation cephalosporin that is FDA approved for the treatment of mild to moderate infections of upper respiratory tract, lower respiratory tract, and uncomplicated skin and Skin-structure infections. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cefprozil tablets are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
Upper respiratory tract
- Pharyngitis/tonsillitis caused by Streptococcus pyogenes.
- Dosage: 500 mg daily for 10 days
- Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
- Dosage: 500 mg each 12 hours for 10 days
- Acute Sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
- Dosage: 250 mg each 12 hours for 10 days
Lower respiratory tract
- Secondary Bacterial Infection of Acute Bronchitis and Acute Bacterial Exacerbation of Chronic Bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
- Dosage: 500 mg each 12 hours for 10 days
Uncomplicated Skin and Skin-Structure Infections
- Caused by Staphylococcus aureus (including penicillinase-producing strains) and Streptococcus pyogenes. Abscesses usually require surgical drainage.
- 250 mg each 12 hours for 10 days, or
- 500 mg daily for 10 days, or
- 500 mg each 12 hours for 10 days
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefprozil in adult patients.
Non–Guideline-Supported Use
- Osteomyelitis
- Pneumonia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Cefprozil tablets are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
Upper respiratory tract
- Pharyngitis/tonsillitis caused by Streptococcus pyogenes.
- Dosage for children between 2-12 years: 7.5 mg/kg daily for 110 days
- Otitis Media caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
- Dosage for 6 month - 12 years: 15 mg/kg each 12 hours for 10 days.
- Acute Sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
- Dosage for 6 months - 12 years: 7.5 mg/kg for 10 days or 15 mg/kg each 12 hours for 10 days.
Lower respiratory tract
- Secondary Bacterial Infection of Acute Bronchitis and Acute Bacterial Exacerbation of Chronic Bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae (including β-lactamase-producing strains), and Moraxella (Branhamella) catarrhalis (including β-lactamase-producing strains).
Uncomplicated Skin and Skin-Structure Infections
- Caused by Staphylococcus aureus (including penicillinase-producing strains) and Streptococcus pyogenes. Abscesses usually require surgical drainage.
- Dosage for children between 2-12 years: 20 mg/kg daily for 10 days.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefprozil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefprozil in pediatric patients.
Contraindications
Cefprozil tablets are contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
Warnings
BEFORE THERAPY WITH CEFPROZIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPROZIL, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-SENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPROZIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefprozil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
The most common adverse effects observed in patients treated with cefprozil are:
- Gastrointestinal: Diarrhea (2.9%), nausea (3.5%), vomiting (1%), and abdominal pain (1%).
- Hepatobiliary: Elevations of AST (SGOT) (2%), ALT (SGPT) (2%), alkaline phosphatase (0.2%), and bilirubin values (<0.1%). As with some penicillins and some other cephalosporin antibiotics, cholestatic jaundice has been reported rarely.
- Hypersensitivity: Rash (0.9%), urticaria (0.1%). Such reactions have been reported more frequently in children than in adults. Signs and symptoms usually occur a few days after initiation of therapy and subside within a few days after cessation of therapy.
- CNS: Dizziness (1%). hyperactivity, headache, nervousness, insomnia, confusion, and somnolence have been reported rarely (<1%). All were reversible.
- Hematopoietic: Decreased leukocyte count (0.2%), eosinophilia (2.3%).
- Renal: Elevated BUN (0.1%), serum creatinine (0.1%).
- Other: Diaper rash and superinfection (1.5%), genital pruritus and vaginitis (1.6%).
Cephalosporin class paragraph
In addition to the adverse reactions listed above which have been observed in patients treated with cefprozil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Aplastic anemia, hemolytic anemia, hemorrhage, renal dysfunction, toxic epidermal necrolysis, toxic nephropathy, prolonged prothrombin time, positive Coombs’ test, elevated LDH, pancytopenia, neutropenia, agranulocytosis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Postmarketing Experience
The following adverse events, regardless of established causal relationship to cefprozil tablets, have been rarely reported during postmarketing surveillance: anaphylaxis, angioedema, colitis (including pseudomembranous colitis), erythema multiforme, fever, serum-sickness like reactions, Stevens-Johnson syndrome, and thrombocytopenia.
Drug Interactions
- Nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporin antibiotics. Concomitant administration of probenecid doubled the AUC for cefprozil.
- The bioavailability of the capsule formulation of cefprozil was not affected when administered 5 minutes following an antacid.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in rabbits, mice, and rats using oral doses of cefprozil of 0.8, 8.5, and 18.5 times the maximum daily human dose (1000 mg) based upon mg/m2, and have revealed no harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefprozil in women who are pregnant.
Labor and Delivery
Cefprozil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
Nursing Mothers
Small amounts of cefprozil (<0.3% of dose) have been detected in human milk following administration of a single 1 gram dose to lactating women. The average levels over 24 hours ranged from 0.25 to 3.3 mcg/mL. Caution should be exercised when cefprozil tablets are administered to a nursing woman, since the effect of cefprozil on nursing infants is unknown.
Pediatric Use
The safety and effectiveness of cefprozil in the treatment of otitis media have been established in the age groups 6 months to 12 years. Use of cefprozil tablets for the treatment of otitis media is supported by evidence from adequate and well-controlled studies of cefprozil in pediatric patients.
The safety and effectiveness of cefprozil in the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections have been established in the age groups 2 to 12 years. Use of cefprozil for the treatment of these infections is supported by evidence from adequate and well-controlled studies of cefprozil in pediatric patients.
The safety and effectiveness of cefprozil in the treatment of acute sinusitis have been established in the age groups 6 months to 12 years. Use of cefprozil in these age groups is supported by evidence from adequate and well-controlled studies of cefprozil in adults.
Safety and effectiveness in pediatric patients below the age of 6 months have not been established for the treatment of otitis media or acute sinusitis, or below the age of 2 years for the treatment of pharyngitis/tonsillitis or uncomplicated skin and skin-structure infections. However, accumulation of other cephalosporin antibiotics in newborn infants (resulting from prolonged drug half-life in this age group) has been reported.
Geriatic Use
Of the more than 4500 adults treated with cefprozil tablets in clinical studies, 14% were 65 years and older, while 5% were 75 years and older. When geriatric patients received the usual recommended adult doses, their clinical efficacy and safety were comparable to clinical efficacy and safety in nongeriatric adult patients. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals to the effects of cefprozil tablets cannot be excluded.
Cefprozil is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Cefprozil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cefprozil with respect to specific racial populations.
Renal Impairment
In patients with known or suspected renal impairment, careful clinical observation and appropriate laboratory studies should be done prior to and during therapy. The total daily dose of cefprozil tablets should be reduced in these patients because high and/or prolonged plasma antibiotic concentrations can occur in such individuals from usual doses. Cephalosporins, including cefprozil tablets, should be given with caution to patients receiving concurrent treatment with potent diuretics since these agents are suspected of adversely affecting renal function.
Hepatic Impairment
In patients with impaired hepatic function, the half-life increases to approximately 2 hours. The magnitude of the changes does not warrant a dosage adjustment for patients with impaired hepatic function.
Females of Reproductive Potential and Males
Impairment of fertility was not observed in male or female rats given oral doses of cefprozil up to 18.5 times the highest recommended human dose based upon mg/m2.
Immunocompromised Patients
There is no FDA guidance one the use of Cefprozil in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Cefprozil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cefprozil and IV administrations.
Overdosage
Single 5000 mg/kg oral doses of cefprozil caused no mortality or signs of toxicity in adult, weanling, or neonatal rats, or adult mice. A single oral dose of 3000 mg/kg caused diarrhea and loss of appetite in cynomolgus monkeys, but no mortality.
Cefprozil is eliminated primarily by the kidneys. In case of severe overdosage, especially in patients with compromised renal function, hemodialysis will aid in the removal of cefprozil from the body.
Pharmacology
| |
Cefprozil
| |
| Systematic (IUPAC) name | |
| 7-[2-amino-2-(4-hydroxyphenyl)-acetyl]amino-8-oxo-3-prop-1-enyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 389.427 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 36% |
| Metabolism | ? |
| Half life | 1.3 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Cefprozil has in vitro activity against a broad range of gram-positive and gram-negative bacteria. The bactericidal action of cefprozil results from inhibition of cell-wall synthesis.
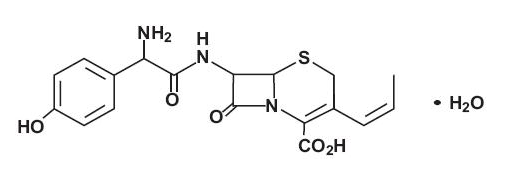
Structure
The structural formula is:
Pharmacodynamics
There is limited information regarding Cefprozil Pharmacodynamics in the drug label.
Pharmacokinetics
The pharmacokinetic data were derived from the capsule formulation; however, bioequivalence has been demonstrated for the oral solution, capsule, tablet, and suspension formulations under fasting conditions.
Following oral administration of cefprozil to fasting subjects, approximately 95% of the dose was absorbed. The average plasma half-life in normal subjects was 1.3 hours, while the steady-state volume of distribution was estimated to be 0.23 L/kg. The total body clearance and renal clearance rates were approximately 3 mL/min/kg and 2.3 mL/min/kg, respectively.
Average peak plasma concentrations after administration of 250 mg, 500 mg, or 1 g doses of cefprozil to fasting subjects were approximately 6.1, 10.5, and 18.3 mcg/mL, respectively, and were obtained within 1.5 hours after dosing. Urinary recovery accounted for approximately 60% of the administered dose.
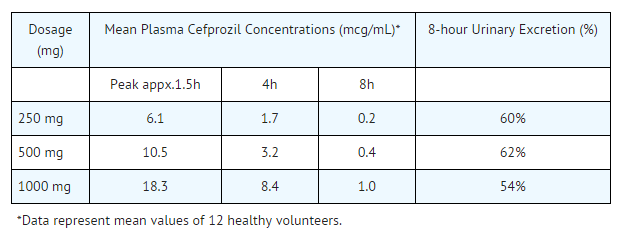
During the first 4-hour period after drug administration, the average urine concentrations following 250 mg, 500 mg, and 1 g doses were approximately 700 mcg/mL, 1000 mcg/mL, and 2900 mcg/mL, respectively.
Administration of cefprozil with food did not affect the extent of absorption (AUC) or the peak plasma concentration (Cmax) of cefprozil. However, there was an increase of 0.25 to 0.75 hours in the time to maximum plasma concentration of cefprozil (Tmax).
The bioavailability of the capsule formulation of cefprozil was not affected when administered 5 minutes following an antacid.
Plasma protein binding is approximately 36% and is independent of concentration in the range of 2 mcg/mL to 20 mcg/mL.
There was no evidence of accumulation of cefprozil in the plasma in individuals with normal renal function following multiple oral doses of up to 1000 mg every 8 hours for 10 days.
Nonclinical Toxicology
Drug/Laboratory Test Interactions
Cephalosporin antibiotics may produce a false positive reaction for glucose in the urine with copper reduction tests, but not with enzyme-based tests for glycosuria. A false negative reaction may occur in the ferricyanide test for blood glucose. The presence of cefprozil in the blood does not interfere with the assay of plasma or urine creatinine by the alkaline picrate method.
Carcinogenesis and Mutagenesis
Long term in vivo studies have not been performed to evaluate the carcinogenic potential of cefprozil.
Cefprozil was not found to be mutagenic in either the Ames Salmonella or E. coli WP2 urvA reversion assays or the Chinese hamster ovary cell HGPRT forward gene mutation assay and it did not induce chromosomal abnormalities in Chinese hamster ovary cells or unscheduled DNA synthesis in rat hepatocytes in vitro. Chromosomal aberrations were not observed in bone marrow cells from rats dosed orally with over 30 times the highest recommended human dose based upon mg/m2.
Clinical Studies
Study One
In a controlled clinical study of acute otitis media performed in the United States where significant rates of β-lactamase-producing organisms were found, cefprozil was compared to an oral antimicrobial agent that contained a specific β-lactamase inhibitor. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10 to 16 days post-therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes i.e., clinical success) and safety results were obtained:

Safety
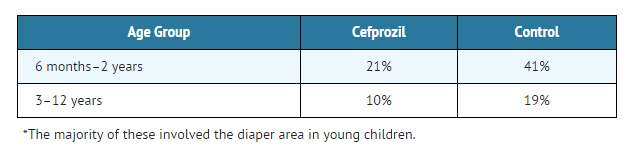
The incidences of adverse events, primarily diarrhea and rash*, were clinically and statistically significantly higher in the control arm versus the cefprozil arm.
Study Two
In a controlled clinical study of acute otitis media performed in Europe, cefprozil was compared to an oral antimicrobial agent that contained a specific β-lactamase inhibitor. As expected in a European population, this study population had a lower incidence of β-lactamase-producing organisms than usually seen in U.S. trials. In this study, using very strict evaluability criteria and microbiologic and clinical response criteria at the 10 to 16 days post-therapy follow-up, the following presumptive bacterial eradication/clinical cure outcomes (i.e., clinical success) were obtained:
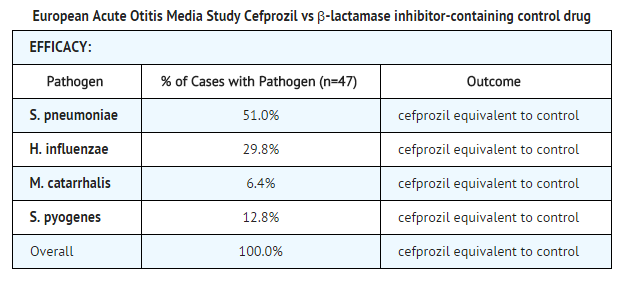
Safety
The incidence of adverse events in the cefprozil arm was comparable to the incidence of adverse events in the control arm (agent that contained a specific β-lactamase inhibitor).
How Supplied
- Cefprozil Tablets 250 mg
- Bottles of 100
- NDC 57237-036-01
- Cefprozil Tablets 500 mg
- Bottles of 50
- NDC 57237-037-50
Bottles of 100 NDC 57237-037-01
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Cefprozil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
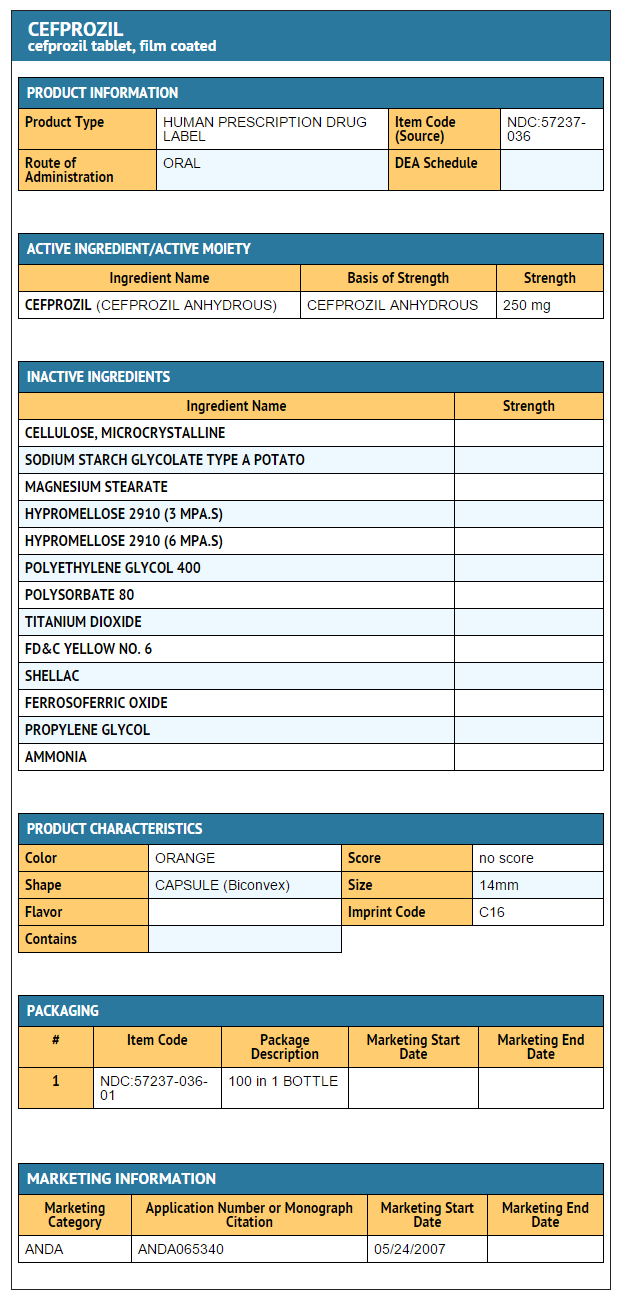
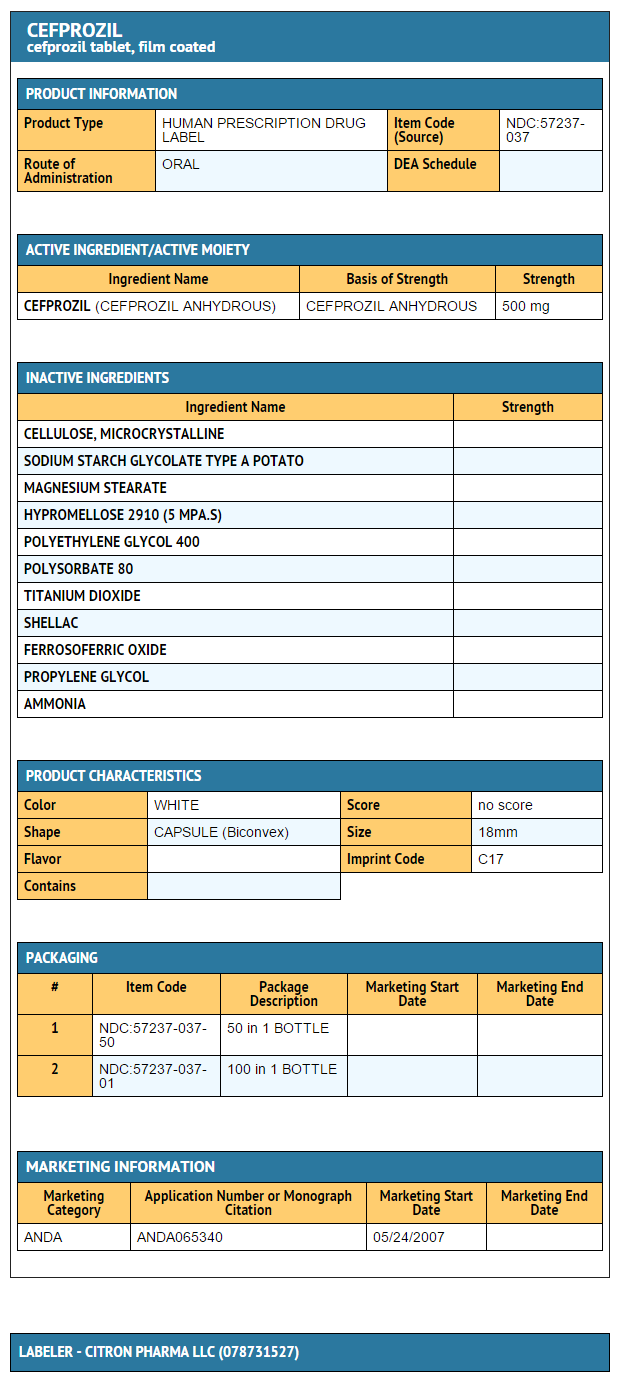
{{#ask: Label Page::Cefprozil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be counseled that antibacterial drugs including cefprozil tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefprozil tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefprozil tablets or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
Alcohol-Cefprozil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cefzil
Look-Alike Drug Names
There is limited information regarding Cefprozil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [4]
Overview
Synonyms: Cefprozilo [INN-Spanish], Cefprozil anhydrous, Cefprozilum [INN-Latin]. A second generation Cephalosporins .It comes either tablet or powder as oral suspension.
Category
Cephalosporins,second generation.
US Brand Names
Cefzil®, Cefprozil®.
Dosing and Administration
Cefprozil tablets and oral suspension are administered orally.
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050664s024,050665s024lbl.pdf