Levothyroxine detailed information
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Mainly in liver, kidneys, brain and muscles |
| Elimination half-life | ca. 7 days (in hyperthyroidism 3-4 days, in hypothyroidism 9-10 days) |
| Excretion | Through feces and urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
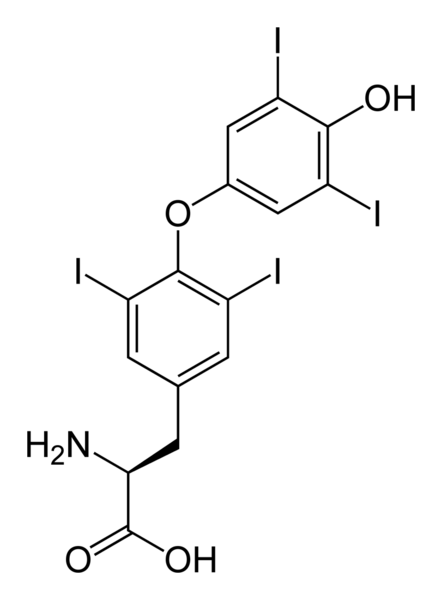
| Formula | C15H10I4NNaO4 |
| Molar mass | 798.86 (anhydrous) |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Levothyroxine, also known as L-thyroxine, synthetic T4 or 3,5,3',5'-tetraiodo-L-thyronine, is a synthetic form of thyroxine (thyroid hormone). The natural hormone is chemically in the L-form, as is the pharmaceutical agent. Dextrothyroxine (D-thyroxine) briefly saw research as an anticholesterol agent but was pulled due to cardiac side-effects.
The EU has recently standardized the use of the name "levothyroxine" for the drug. Common brand names include ""Thyrax"", "Euthyrox", "Levaxin" and "Eltroxin" in Europe, and "Levoxyl" and "Synthroid" in the U.S. There are also numerous generic versions approved by the Food and Drug Administration.
Uses
This medicine is a hormone replacement usually given to patients with thyroid problems, specifically, hypothyroidism. It is also given to people who have Goitre or an enlarged thyroid gland.
Administration
It is recommended that levothyroxine be taken half an hour to an hour before meals to maximize its absorption. It is also recommended that the patient take the tablet with one glass of water to ease swallowing as well as to help the tablet dissolve for absorption.
Precautions and side effects
There are also foods and other substances that can interfere with absorption of thyroxine replacement. Avoid taking calcium and iron supplements within 4 hours of the medication and avoid taking soy products within 3 hours of the medication as these can reduce absorption of the medication.
Synthetic levothyroxine may have adverse side effects like: palpitations, nervousness, headache, difficulty sleeping, insomnia, swelling of the legs and ankles, weight loss and/or increased appetite. Some may be allergic to the medicine. If the patient develops a severe reaction to this drug like difficulty breathing, shortness of breath or swelling of the face and tongue it is imperative that the patient immediately seek medical attention.
Marketing and approvals
Synthroid is the most prescribed brand of T4 in United States. Synthroid was marketed in 1955,[1] but was not FDA approved at that time as it was considered "generally regarded safe". In the 1990s, in response to debate as to whether Synthroid was more effective than other levothyroxine preparations, (which ended up concluding that there was little difference between Synthroid and generic brands[2] all levothyroxine preparations were required to undergo the formal FDA approval process. Synthroid was approved by the FDA on 2002-07-24.[3]
References
External links
- Detailed Euthyrox (Levothroid/Levothyroxine) Consumer Information: Uses, Precautions, Side Effects
- Leaflet
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Hormonal agents
- Iodinated tyrosine derivatives
- Endocrinology